Noninvasive prenatal testing: benefits and limitations of the available tests
Neinvazivní prenatální testy: jejich přínos a limity
Úvod: Za „zlatý standard“ pro prenatální diagnostiku aneuploidií je považováno vyšetření karyotypu, které má vysokou přesnost, ale je závislé na invazivních metodách, které nesou riziko těhotenské ztráty. Pro klinické využití byly k dispozici různé neinvazivní prenatální genetické testy (NIPT), které zahrnovaly metody pro sledování aneuploidií, včetně pohlavních chromozomů, metody pro vyloučení otcovství a některé mikrodelece a triploidie.Tyto specifické testy využívají tři metodické nástroje: s-MPS, t-MPS a SNP. Genetické testy, navzdory vysokým nákladům, pokrývají širší škálu klinických aplikací a mají tu výhodu, že mohou být provedeny časně, s vysokou přesností a nízkou falešnou pozitivitou.
Typ studie: Přehledový článek.
Název a sídlo pracoviště: Gynekologicko-porodnická klinika, Přírodovědecká fakulta Santa Casa v São Paulu (FSMSCSP), São Paulo-SP, Brazílie.
Metodika: Tato studie využívala PubMed/MEDLINE jako zdroj výzkumu a zaměřila se na shromažďování údajů, které umožňovaly sledovat vývoj, technické a metodologické pokroky dostupných testů, posoudit jeho přínos, omezení a budoucí perspektivy na NIPT.
Závěr: NIPT vyniká tím, že může být využito v časné fázi těhotenství s vysokou přesností a nízkou falešnou pozitivitou, včetně širokého spektra klinického využití. T-MPS je nejnovější technika používaná k vyhodnocení aneuploidií, která se vyznačuje větší přesností a nižšími náklady než s-MPS, ale je omezena pouze na nejběžnější aneuploidie. Technika SNP může hledat více genetických mutací s větší přesností.
Klíčová slova:
prenatální diagnostika – genetický test – DNA/krev – onemocnění plodu
Authors:
M. V. C. Andari 1; S. L. C. Bussamra 1,2; T. G. D. Tedesco 1; P. A. B. Peixoto 2,3,4; P. D. B. S. Pares 2; Antonio Braga 5
; Edward Araujo Júnior 2
; T. Aoki 1
Authors‘ workplace:
Department of Obstetrics and Gynecology, Science College of Santa Casa of São Paulo (FSMSCSP), São Paulo-SP, Brazil
1; Department of Obstetrics, Paulista School of Medicine – Federal University of São Paulo (EPM-UNIFESP), São Paulo-SP, Brazil
2; Mário Palmério University Hospital, University of Uberaba (UNIUBE), Uberaba-MG, Brazil
3; Department of Obstetrics and Gynecology, Federal University of Triângulo Mineiro (UFTM), Uberaba-MG, Brazil
4; Department of Obstetrics and Gynecology, Federal University of Rio de Janeiro (UFRJ), Rio de Janeiro, Brazil
5
Published in:
Ceska Gynekol 2020; 85(1): 41-48
Category:
Review Article
Overview
Background: The „gold standard“ for prenatal diagnosis of aneuploidies is provided by the karyotype, which has high accuracy, but is dependent on invasive procedures, which generate risk of fetal loss. Different methodologies of development of noninvasive prenatal genetic tests (NIPT) for tracking aneuploidies, including sex chromosomes, have been made available for clinical use, for some microdeletions and triploids and for exclusion of paternity. These exams make use of three methodological tools: s-MPS, t-MPS and SNP. Genetic tests, despite the high cost, cover a broader range of clinical applications, have the advantage that can be performed early, with high accuracy, and low false positive rate.
Type of article: Review.
Setting: Department of Obstetrics and Gynecology, Science College of Santa Casa of São Paulo (FSMSCSP), São Paulo-SP, Brazil.
Design and methods: This study was a non-asystematic review, which searched PubMed / MEDLINE as a research source and aimed at the compilation of data, which allowed approaching the evolution, the technical and methodological advances of the available tests, the recognition of its benefits, limitations and future perspectives on NIPT.
Conclusion: NIPT stand out for being applied earlier during the pregnancy with high accuracy and low false-positive rates, including a broad spectrum of clinical applications. The t-MPS is a recent technique used to evaluate aneuploidy that shows greater accuracy and lower cost than the s-MPS, but that is limited to being applied only to the most common aneuploidies. The SNP technique can search for more genetic conditions, besides presenting better accuracy.
Keywords:
prenatal diagnosis – genetic test – DNA/blood – fetal diseases
INTRODUCTION
Aneuploidies are the most common chromosomal abnormalities. The most frequent are trisomy of chromosome 21 (Down syndrome) – 53%; 18 (Edwards’ syndrome) – 13%; 13 (Patau syndrome) – 5%; monosomy of chromosome X (Turner syndrome) – 8%; and trisomy of sex chromosomes (XXX – triple X syndrome; XXY – Klinefelter syndrome; XYY – Jacob’s syndrome) – 4% [46, 55].
Chromosomal abnormalities are diagnosed in the prenatal stage using invasive tests. Chorionic villus sampling (CVS) and amniocentesis are the two most important tests, but both present a risk of fetal loss of about 0.1% to 0.5% [1, 49]. Since there are risks, invasive tests should be reserved for situations of high risk for aneuploidies. During pregnancy, screening tests for the most common trisomies can be applied universally, since they represent no risks to the mother or fetus [1].
Currently, the screening of chromosomal abnormalities during the first trimester of pregnancy is a combined test involving the evaluation of maternal age and ultrasound and biochemical markers. The combined test raises trisomy 21 detection rates to 95% with a false-positive rate of 2% [35]. In recent years, a new screening method was developed to detect the main chromosomal abnormalities (trisomy 21, 18 and 13) based on the screening of free fetal DNA fragments in the maternal blood (c DNA – cell free fetal DNA) [41,43]. The detection rates are higher than any other screening methods used. Recent studies showed that DNA tests can detect about 99% of trisomy 21 cases, 98% of trisomy 18 cases, and 99% of trisomy 13 cases, with combined false-positive rates of 0.13% [41,43].
The screening of free fetal DNA fragments in the maternal blood is a modality of noninvasive prenatal testing (NIPT) that can be clinically used to screen aneuploidies and diagnose many other prenatal disorders, minimizing risks and the need for invasive procedures. NIPT also help in the study of sex-related diseases and in the detection of maternal-fetal isoimmunization through the determination of fetal sex and RH genotyping, respectively [17, 33]. It can also be used to differentiate fetal genetic information in order to solve and diagnose monogenic diseases [3–16]. Another use of NIPT is the possibility of excluding paternity through maternal blood, with an accuracy of over 99% [42].
The use of NIPT during prenatal care has been a subject of interest not only to the scientific and medical communities, but also to the media and patients. Many publications involving NIPT modified the global scenario of prenatal testing and motivated the search for updating and information. Thus, a literature review on the development and technical and methodological advances of the available tests was conducted to recognize benefits, limitations and future perspectives of NIPT.
METHODS
This study is a non-systematic literature review to search for scientific papers in English on the PubMed/MEDLINE database, the US National Library of Medicine, and the US National Institutes of Health. The reference search did not limit the search period by date of publication.
EVOLUTION OF ANEUPLOIDY SCREENING METHODS
The first screening method for risk of fetal chromosomal abnormalities, introduced in the early 1970s, was based only on advanced maternal age, defined as older than 35. At that time, the risk group included 5% of the pregnant population, 30% of fetuses with Down syndrome, and only 2% of the mothers undergoing invasive diagnoses presented a disorder [9, 37].
In the following decade, many fetal-placental products were concentrated in the second trimester of pregnancy – in the form of double, triple and quadruple tests – and combined with advanced maternal age, significantly increasing the accuracy of aneuploidy screening. The maternal serum QUAD screening measures the levels of four biochemical markers in the maternal blood: α-fetoprotein, human chorionic β-gonadotropin (β-HCG), unconjugated estriol, and inhibin A, providing an adjusted calculation for risk of aneuploidies associated with maternal age, with a detection rate of 80% for trisomies of chromosomes 21 and 18 and a false-positive rate of 5% [53].
In the 1990s, Down Syndrome screening was introduced by combining maternal age with the ultrasound measurement of nuchal translucency (NT) during the first trimester of pregnancy. This tool proved to be effective in identifying 75–80% of the fetuses with chromosome changes, with a false-positive rate of approximately 5% [36]. Subsequently, the parameters maternal age and NT measurement were associated with the biochemical testing of two maternal hormones – the free β-HCG and the pregnancy-associated plasma protein A (PAPP-A) in the form of combined tests for trisomy 21 screening during the first trimester of pregnancy. Based on these results, the patient could analyze the adjusted risk of carrying an aneuploid fetus, with a detection rate of 90% for Down syndrome and over 80% for Edwards’ syndrome, and a false-positive rate of de 5% [19]. The inclusion of additional parameters, such as nasal bone measurement, facial angle, and tricuspid and venous duct Doppler, increases the identification rate of trisomy 21 to 95% and decreases the false-positive rate to 2% [35].
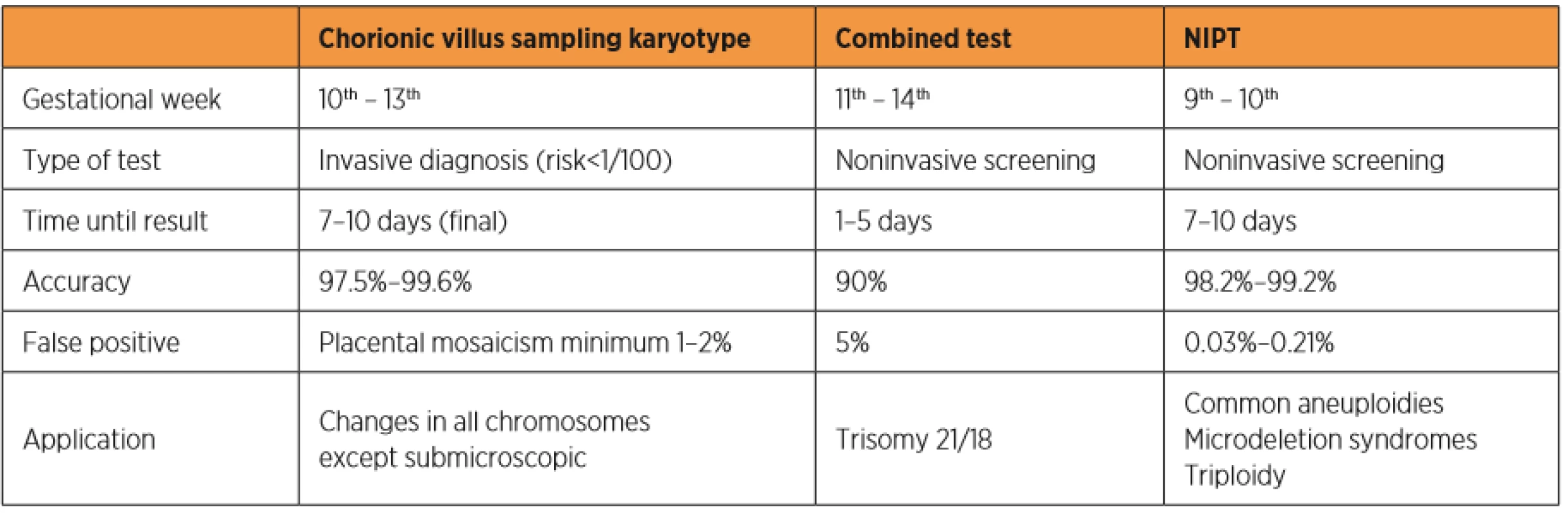
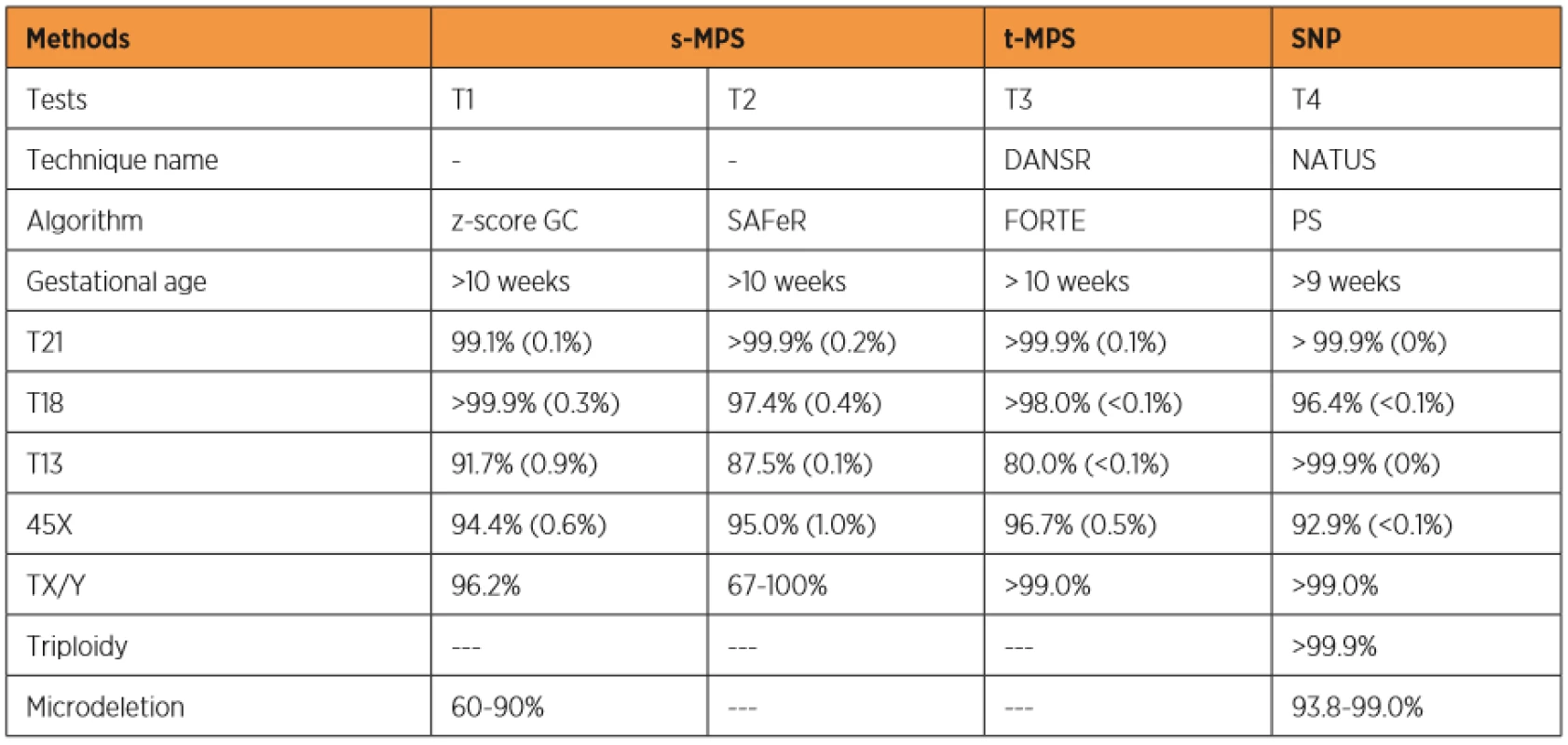
In recent years, a new screening method for trisomy of chromosomes 21, 18 and 13 was developed based on research on free fetal DNA fragments in maternal blood (c DNA – cell free fetal DNA) [26]. Some studies showed that both intact fetal cells and c DNA cross the placenta and reach the maternal circulation [6, 26]. The release of free fetal DNA into maternal plasma possibly occurs due to the continuous escape of fetal cells through the placenta, which are immediately destroyed by the maternal immune systém [38]. Free fetal DNA is present in maternal circulation after the fourth week and after the tenth week of gestation it represents more than 4% of all free DNA [18]. Since fetal DNA is undetectable within 2 hours postpartum, a prenatal test based on c DNA is not influenced by previous pregnancies [18]. A recent study shows that DNA tests can detect near 99% of trisomy 21, 98% of trisomy 18, and 99% of trisomy 13 cases with combined false-positive rates of 0.13% [41]. These detection rates are higher than in any other screening method used. Table 1 shows a comparison of prenatal tests for aneuploidies available during the first trimester of pregnancy.
TYPES OF GENETIC NONINVASIVE PRENATAL TESTING (NIPT)
During the last decades, some studies explored the possibility of developing NIPT methods to analyze fetal genetic material using maternal blood during the first trimester of pregnancy [24]. During pregnancy, the circulatory system of the mother and the fetus is separated by the placental membrane; however, some evidence indicated the existence of bidirectional maternal-fetal trafficking [29]. Some studies reported that both intact fetal cells and c DNA cross the placenta and reach the maternal circulation [6, 26].
Although most human DNA is located inside cells, there is a small quantity of extracellular DNA in the circulation of healthy and unhealthy people [38]. The release of free fetal DNA into maternal plasma possibly occurs due to the continuous escape of fetal cells through the placenta, which are immediately destroyed by the maternal immune syste [38]. These free fetal molecules are detected in maternal blood after seven weeks of pregnancy, but can be identified in the maternal circulation after the forth gestational week [18]. The concentration of fetal molecules increases with gestational age, peaking in the last eight weeks of pregnancy [31]. They are quickly eliminated from the maternal circulation, with a half-life of 16.3 minutes, which makes them undetectable two hours after delivery [28]. Lo reported a mean concentration of c DNA fraction for the first, second and third trimesters of 9.7%, 9.0% and 20.4% of total maternal plasma DNA, respectively [24].
In general, clinical applications of prenatal diagnosis using maternal blood focus on the quantitative measurement of c DNA concentration and on the qualitative detection of specific sequences or genes of these molecules. There are some challenges associated with noninvasive diagnostic tests using maternal blood because the concentration of all free DNA is relatively low in maternal blood, the total quantity of DNA varies between individuals, there are 20 times more free maternal DNA molecules than c DNA molecules in maternal blood, and the fetus inherits and shares half of the maternal genome [56].
APPLICATION OF GENETIC NONINVASIVE PRENATAL TESTING
Determination of fetal sex
Detection and quantification of fetal DNA in maternal plasma usually involve quantification systems that use Y-derived chromosome sequences which are absent in the maternal genotype and, therefore, paternally inherited by the fetus. These systems are sensitive enough to detect fetal DNA equivalent to a single cell [33]. In a representative study with patients between 7-16 gestational weeks, Sekizawa et al [47] demonstrated 97.2% sensitivity and 100% specificity using PCR technique. Akolekar et al. [2] analyzed the accuracy of fetal sex detection using a spectrometry-based platform with three Y chromosome sequences (SRY, DBY and TTTY2) at the same time during the first trimester of pregnancy. They reported 99.1% accuracy, 98.9% sensitivity, and 99.2% specificity.
Fetal sex determination is also used as a diagnostic tool when a male fetus is at risk of being affected by sex-related genetic diseases, such as: Duchenne muscular dystrophy; hemophilia; X-linked mental retardation, immunodeficiency and hydrocephalus; adrenoleukodystrophy; retinitis pigmentosa; and Hunter, Menkes and Lesch-Nyhan, Alport syndromes [8]. This diagnostic proposal is also applied to the study of congenital adrenal hyperplasia, which causes ambiguous external development of the genitalia and masculinization of female fetuses. Determining a female fetus allows the administration of dexamethasone to the mother before the ninth week to prevent consequent fetal virilization [45].
Fetal RHD genotyping test
Noninvasive prenatal diagnosis to determine fetal RHD genotype in Rh-negative women was first demonstrated by Lo et al. [27]. This procedure can minimize unnecessary prophylactic immunization, in addition to reducing the risk of contamination during diagnosis [52].
A meta-analysis reported that fetal RHD genotyping tests in maternal blood using the c DNA technique in maternal plasma and serum had 96.1% sensitivity and 96.5% specificity. The genotype was detected in the first trimester [13].
The need of examining more than one region of the RHD gene to obtain Rh typing was suggested due to the high complexity of the Rh system and the possibility of false-positive results [4]. Aykut et al. [5] analyzed the specific exons 7 and 10 of the RHD gene in the blood of pregnant Rh-negative women between 9–39 weeks of pregnancy and reported 100% sensitivity and specificity.
Monogenic diseases
The use of NIPT to evaluate monogenic disorders is part of the clinical practice in medical genetics, especially when there is family history of a specific disease [22]. To date, NIPT has been directed to the analysis of de novo (new) or paternally inherited mutations in order to ensure fetal origin [7].
C DNA has been used to detect several genetic diseases, such as myotonic dystrophy [3], achondroplasia [23], cystic fibrosis [14] and Huntington’s disease [15]. Monogenic diseases with autosomal recessive inheritance are difficult to detect because in these cases both the mother and the father carry mutations and the fetus will be affected if inheriting both mutations. The diagnosis becomes complex when the couple has identical mutations, since the fetus and the mother have the same alleles and they are indistinguishable in the plasma [56].
Aneuploidies
In 1999, researchers showed that pregnant women with Down syndrome fetuses had a higher absolute c DNA concentration in their plasma and serum than in pregnancies with euploid fetuses [28]. They also observed it in pregnant women with fetuses with chromosome 13 trisomy, but not with fetus with chromosome 18 trisomy [54]. Subsequently, diagnostic strategies were focused on detecting and quantifying specific markers of the chromosomes involved in the most common aneuploidies. In 2000, Poon et al. [40] detected mRNA transcribed from the Y chromosome in the plasma of pregnant women with male fetuses. A tool for the systematic identification of fetal mRNA markers of maternal plasma based on microarrays was developed in 2004 [51]. This tool can detect and quantify placental PLAC4 fetal mRNA in chromosome 21, being used for prenatal detection Down syndrome [30].
Another alternative explored was the use of epigenetic markers. An important example of epigenetic studies is the DNA methylation process. In 2002, Poon et al. [39] showed the possibility of developing fetal DNA markers based on different methylation patterns in maternal and fetal tissues by identifying the SERPINB5 gene located on chromosome 18. In 2006, Tong et al. [50] explored the epigenetic differential between fetal and mother DNA and diagnosed chromosome 18 trisomy using the allelic ratio provided by the methylation-specific PCR of the maspin gene promoter in placental tissues.
In 2007, Lo et al. [30] proposed a technical approach based on molecular counting by digital PCR to detect chromosome 21 trisomy, following the basic concept that the greater the number of molecules counted, the greater the discrimination power of the analytical technique. The success of this technique is partly explained by the fact that the proportion of free fetal DNA is higher than what was initially thought [12]. However, the proportion of DNA in the sample still has a strong effect on NIPT reliability for aneuploidies 32].
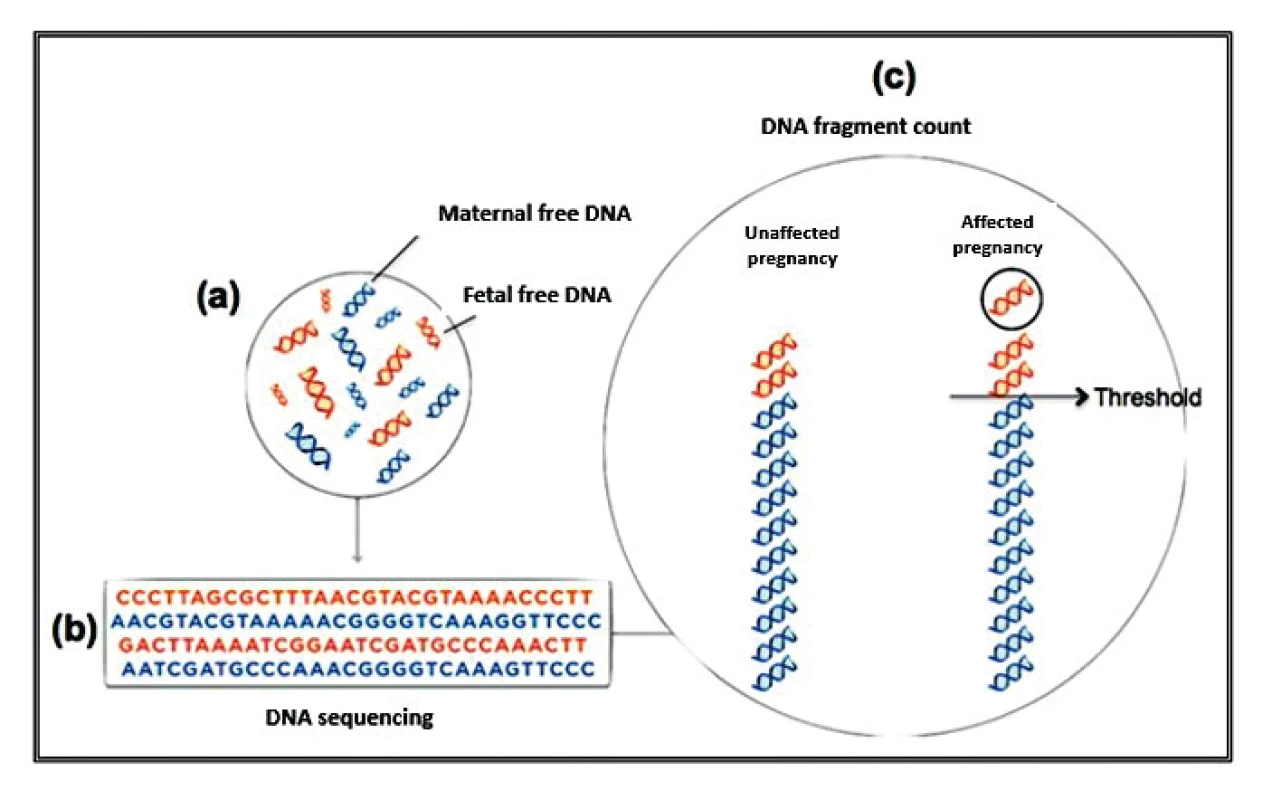
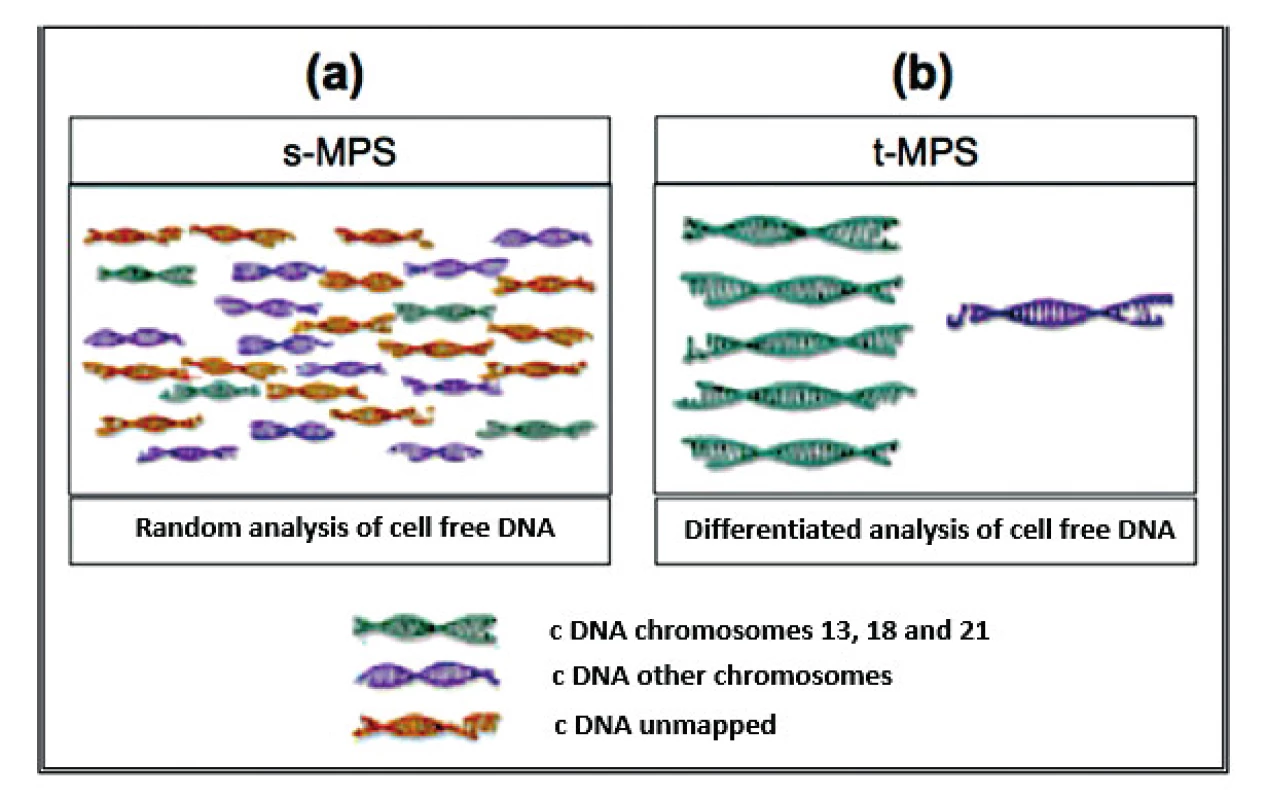
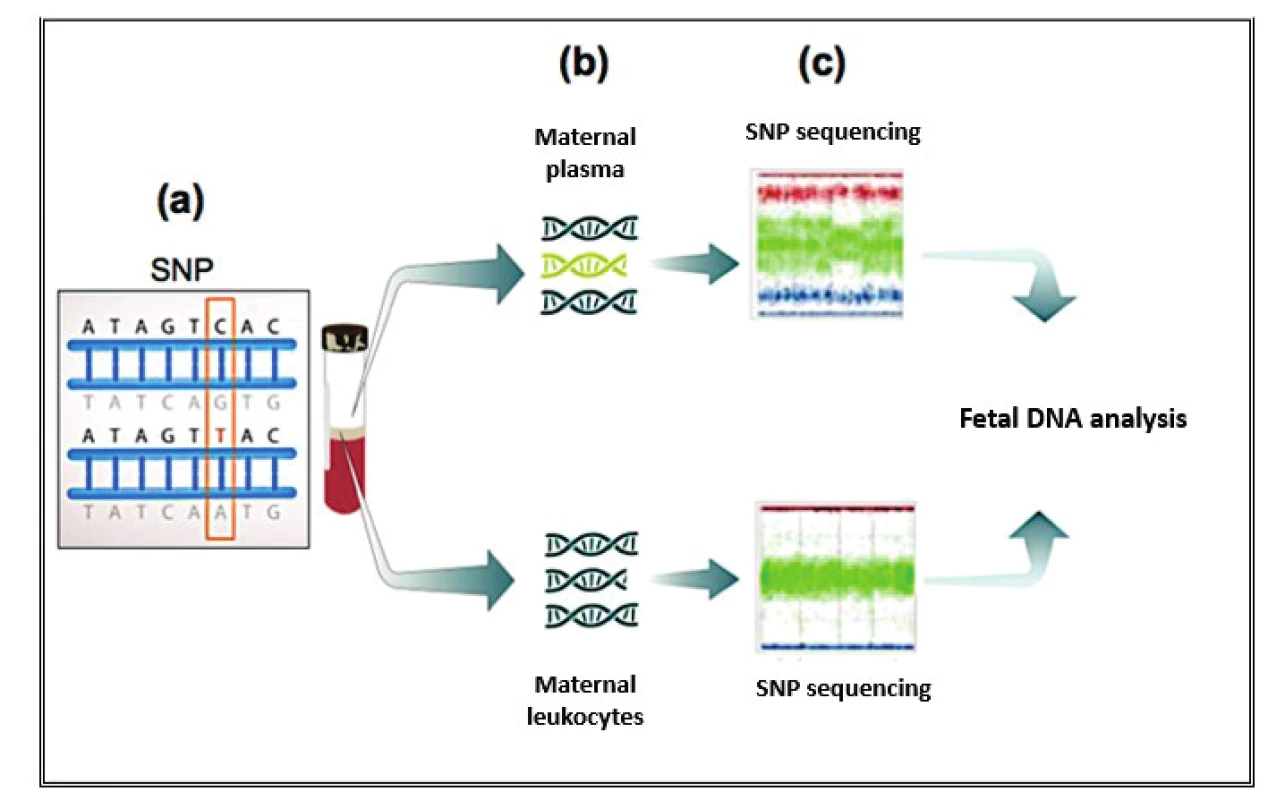
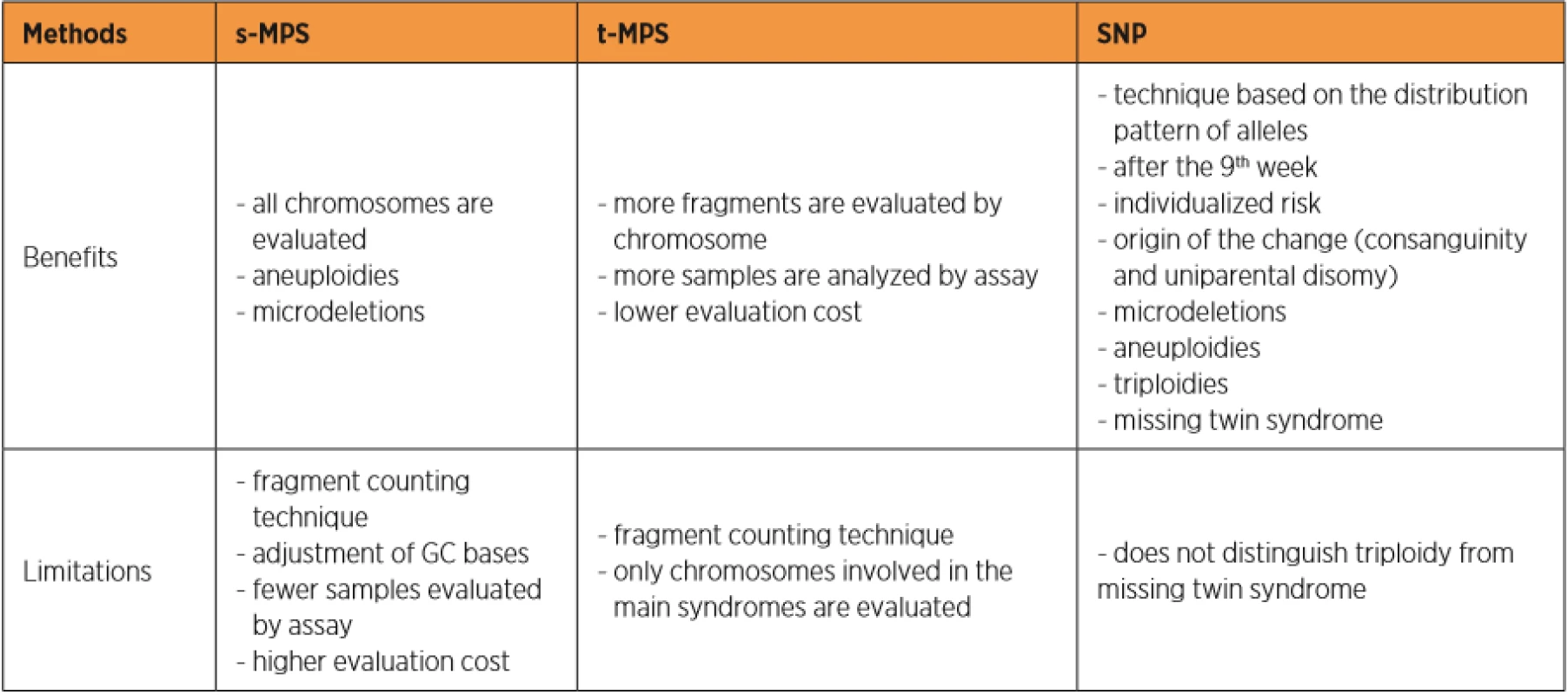
Currently, three different test tools are applied to the NIPT for aneuploidies. The Shotgun Massively Parallel Sequencing (s-MPS), based on the MPS sequencing method, can sequence DNA fragments from the entire genome. It is a technique based on identifying and counting of a large number of DNA fragments from maternal plasma samples. Millions of fetal and maternal DNA fragments (with size of about 25 bp) can be simultaneously sequenced, and, since the complete human genome is known, each sequence mapping a specific locus of that genome can be attributed to the chromosome from which it is originated [10]. The presence of an aneuploidy will result in an excess or deficit related to the studied chromosome compared to what is expected for euploid cases (Figure 1). The second methodology available is the Target Massively Parallel Sequencing (t-MPS). Differently from the s-MPS, which randomly sequences genomic fragments of all chromosomes, the t-MPS selects sequences from genomic regions of interest (e.g., chromosomes 21, 18, 13, X and Y), counts only those sequences and then evaluates when there is a relative excess of one chromosome over another. This tool has the following advantages: low sequencing cost (up to ten times lower) and increased efficiency, since it alternatively counts a greater number of DNA fragments corresponding to specific chromosome regions (Figure 2) [48]. The third NIPT method developed do diagnose aneuploidies is based on the analysis of single nucleotide polymorphisms (SNP) and determines the relative quantitative contribution of maternal and fetal DNA circulating in maternal blood. It is the only method that can distinguish free fetal and maternal DNA. This tool involves the simultaneous amplification of about 20,000 SNP sequences in a single PCR reaction using maternal plasma DNA followed by sequencing. After considering the SNP position in the chromosomes and the possibility of recombination between them, the probability of an euploid, aneuploid or triploid fetus is calculated. It can also identify regions of fetal chromosome homology that indicate consanguinity or uniparental disomy. SNP technology provides information on the origin of aneuploidy, on recombination events and on inherited mutations (Figure 3) [44]. Table 2 presents the benefits and limitations of s-MPS, t-MPS and SNP techniques. Table 3 presents the performance accuracy of s-MPS, t-MPS and SNP methods to detect chromosomal changes.
Whole genome sequencing
The genome of the fetus is the combination of four chromosome lines or haplotypes, two maternal and two paternal, resulting from random arrangements and recombination during cell division [11]. The implementation of Next Generation Sequencing (NGS) technologies in the development of NIPT contributed to increase knowledge in the prenatal area. In 2010, Lo et al. [25] reported the use of NGS techniques to analyze the entire fetal genome for the first time. In this study, the authors concluded that the fetal genome is entirely represented by small c DNA fragments in maternal plasma in a constant relative proportion and suggested that its technical reconstruction was feasible and could detect genetic disorders. In 2012, another group of researchers combined the Whole Genome Sequencing (WGS). The research of maternal genomic haplotypes to demonstrate the WGS of an 18-week fetus showed 98.1% accuracy [20]. Lau et al. [21] reviewed the application of a low coverage WGS platform to NIPT to detect aneuploidies and demonstrated that this procedure presented 100% accuracy in the detection of common trisomies, also allowing the detection of other aneuploidies and structural chromosomal abnormalities with high positive predictive value.
CONCLUSION
In summary, noninvasive prenatal genetic tests stand out for being applied earlier during the pregnancy with high accuracy and low false-positive rates, including a broad spectrum of clinical applications. However these tests are limited by their high cost. The t-MPS is a recent technique used to evaluate aneuploidy that shows greater accuracy and lower cost than the s-MPS, but that is limited to being applied only to the most common aneuploidies. The SNP technique can search for more genetic conditions, besides presenting better accuracy. In the near future, the WGS will represent a generalized method of noninvasive genetic fetal diagnosis that will provide more information on fetal health not only to detect aneuploidies, but a greater number of genetic disorders.
Correspondence:
Prof. Edward Araujo Júnior, PhD
Rua Belchior de Azevedo, 156 apto. 111 Torre Vitoria
São Paulo–SP, Brazil
CEP 05089-030
e-mail: araujojred@terra.com.br
Sources
1. Akolekar, R., Beta, J., Picciarelli, G., et al. Procedure related risk of miscarriage following amniocentesis and chorionic villus sampling: a systematic review and meta-analysis. Ultrasound Obstet Gynecol, 2015, 45(1), p. 16–26.
2. Akolekar, R., Farkas, DH., VanAgtmael, AL., et al. Fetal sex determination using circulating cell-free fetal DNA (ccffDNA) at 11 to 13 weeks of gestation. Prenat Diagn, 2010, 30(10), p. 918–23.
3. Amicucci, P., Gennarelli, M., Novelli, G., Dallapiccola, B. Prenatal diagnosis of Myotonic Dystrophy using fetal DNA obtained from maternal plasma. Clin Chem, 2000, 46(2), p. 301–302.
4. Avent, ND. The Rhesus blood group system: insights from recent advantages in molecular biology. Transfus Med Rev, 1999, 13(4), p. 245–266.
5. Aykut, A., Onay, H., Sagol, S., et al. Determination of fetal Resus D status by maternal plasma DNA Analysis. Balkan J Med Genet, 2013, 16(2), p. 33–38.
6. Bianchi, DW., Flint, AF., Pizzimenti, MF., et al. Isolation of fetal DNA from nucleated erythrocytes in maternal blood. Proc Natl Acad Sci USA, 1990, 87(9), p. 3279–3283.
7. Bustamante-Aragonés, A., Marta Rodríguez-de-Alba, M., Perlado, S., et al. Non-invasive prenatal diagnosis of single-gene disorders from maternal blood. Gene, 2012, 504(1), p. 144–149.
8. Costa, JM., Benachi, A., Gautier, E., et al. [First trimester fetal sex determination in maternal serum using real-time PCR]. Gynecol Obstet Fertil, 2002, 30(12), p. 953–957.
9. Driscoll, DA., Gross, S. Clinical practice. Prenatal screening for aneuploidy. N Engl J Med, 2009, 360(24), p. 2556–2562.
10. Fan, HC., Blumenfeld, YJ., Chitkara, U., et al. Noninvasive diagnosis of fetal aneuploidy by shotgun sequencing DNA from maternal blood. Proc Natl Acad Sci USA, 2008, 105(42), p. 16266–16271.
11. Fan, HC., Gu, W., Wang, J., et al. Non-invasive prenatal measurement of the fetal genome. Nature, 2012, 487(7407), p. 320–324.
12. Fan, HC., Quake, SR. Detection of aneuploidy with digital polymerase chain reaction. Anal Chem, 2007, 79(19), p. 7576–7579.
13. Geifman-Holzman, O., Grotegut, CA., Gaughan, JP. Diagnostic accuracy ofnoninvasive fetal Rh genotyping from maternal blood – a meta-analysis. Am J Obstet Gynecol, 2006, 195(4), p. 1163–1173.
14. Gonzalez-Gonzalez, MC., Garcia-Hoyos, M., Trujillo, MJ., et al. Prenatal detection of a cystic fibrosis mutation in fetal DNA from maternal plasma. Prenat Diagn, 2002, 22(10), p. 946–948.
15. Gonzalez-Gonzalez, MC., Trujillo, MJ., Rodriguez de Alba, M., et al. Huntington disease-unaffected fetus diagnosed from maternal plasma using QF-PCR. Prenat Diagn, 2003, 23(3), p. 232–234.
16. Gu, W., Koh, W., Blumenfeld, YJ., et al. Noninvasive prenatal diagnosis in a fetus at risk for methylmalonic acidemia. Genet Med, 2014, 16(7), p. 564–567.
17. Hill, M., Finning, K., Martin, P., et al. Non-invasive prenatal determination of fetal sex: translating research into clinical practice. Clin Genet, 2011, 80(1), p. 68–75.
18. Illanes, S., Denbow, M., Kailasam, C., et al. Early detection of cell-free fetal DNA in maternal plasma. Early Hum Dev, 2007, 83(9), p. 563–566.
19. Kagan, KO., Wright, D., Baker, A., et al. Screening for trisomy 21 by maternal age, fetal nuchal translucency thickness, free beta-human chorionic gonadotropin and pregnancy-associated plasma protein-A. Ultrasound Obstet Gynecol, 2008, 31(6), p. 618–624.
20. Kitzman, JO., Snyder, MW., Ventura, M., et al. Noninvasive whole-genome sequencing of a human fetus. Sci Transl Med, 2012, 4(137), p. 137ra76.
21. Lau, TK., Cheung, SW., Lo, PS., et al. Non-invasive prenatal testing for fetal chromosomal abnormalities by low-coverage whole-genome sequencing of maternal plasma DNA: review of 1982 consecutive cases in a single center. Ultrasound Obstet Gynecol, 2014, 43(3), p. 254–264.
22. Li, Y., Holzgreve, W., Page-Christiaens, GC., et al. Improved prenatal detection of a fetal point mutation for achondroplasia by the use of size-fractionated circulatory DNA in maternal plasma – case report. Prenat Diagn, 2004, 24(11), p. 896–898.
23. Lim, JH., Kim, MJ., Kim, SY., et al. Non-invasive prenatal detection of achondroplasia using circulating fetal DNA in maternal plasma. J Assist Reprod Genet, 2010, 28(2), p. 167–172.
24. Lo, Y. Noninvasive prenatal detection of fetal chromosomal aneuploidies by maternal plasma nucleic acid analysis: a review of the current state of art. BJOG, 2008, 116(2), p. 152–157.
25. Lo, YM., Chan, KC., Sun, H., et al. Maternal plasma DNA sequencing reveals the genome-wide genetic and mutational profile of the fetus. Sci Transl Med, 2010, 2(61), p. 61–91.
26. Lo, YM., Corbetta, N., Chamberlain, PF., et al. Presence of fetal DNA in maternal plasma and serum. Lancet, 1997, 350(9076), p. 485–487.
27. Lo, YM., Hjelm, NM., Fidler, C., et al. Prenatal diagnosis of fetal RhD status by molecular analysis of maternal plasma. N Engl J Med, 1998, 339(24), p. 1734–1738.
28. Lo, YM., Leung, TN., Tein, MS., et al. Quantitative abnormalities of fetal DNA in maternal plasma in preeclampsia. Clin Chem, 1999, 45(2), p. 184–188.
29. Lo, YM., Lo, ES., Watson, N., Noakes, L., et al. Two-way cell traffic between mother and fetus: biologic and clinical implications. Blood, 1996, 88(11), p. 4390–4395.
30. Lo, YM., Lun, FM., Chan, KC., et al. Digital PCR for the molecular detection of fetal chromosomal aneuploidy. Proc Natl Acad Sci USA, 2007, 104(32), p. 1316–1321.
31. Lo, YM., Tein, MS., Lau, TK., et al. Quantitative analysis of fetal DNA in maternal plasma and serum: implications for non-invasive prenatal diagnosis. Am J Hum Genet, 1998, 62(4), p. 768–775.
32. Lun, FM., Tsui, NB., Chan, KC., et al. Noninvasive prenatal diagnosis of monogenic diseases by digital size selection and relative mutation dosage on DNA in maternal plasma. Proc Natl Acad Sci USA, 2008, 105(50), p. 19920–19925.
33. Martinhago, CD., Oliveira, RM., Canas, MC., et al. Accuracy of fetal gender determination in maternal plasma at 5 and 6 weeks of pregnancy. Prenat Diagn, 2006, 26(13), p. 1219–1223.
34. Newson, AJ. Ethical aspects arising from non-invasive fetal diagnosis. Semin Fetal Neonatal Med, 2008, 13(2), p. 103–108.
35. Nicolaides, K. Some thoughts on the true value of ultrasound. Ultras Obstet Gynecol, 2007, 30(5), p. 671–674.
36. Nicolaides, KH. Nuchal translucency and other first-trimester sonographic markers of chromosomal abnormalities. Am J Obstet Gynecol, 2004, 191(1), p. 45–67.
37. Nicolaides, KH. Screening for fetal aneuploidies at 11 to 13 weeks. Prenat Diagn, 2011, 31(1), p. 7–15.
38. Pertl, B., Sikizawa, A., Samura, O., et al. Detection of male and female fetal DNA in maternal plasma by multiplex fluorescent polymerase chain reaction amplification of short tandem repeats. Hum Genet, 2000, 106(1), p. 45–49.
39. Poon, LL., Leung, TN., Lau, TK., et al. Differential DNA methylation between fetus and mother as a strategy for detecting fetal DNA in maternal plasma. Clin Chem, 2002, 48(1), p. 35–41.
40. Poon, LL., Leung, TN., Lau, TK., Lo, YM. Presence of fetal RNA in maternal plasma. Clin Chem, 2000, 46(11), p. 1832–1834.
41. Quezada, MS., Gil, MM., Francisco, C., et al. Screening for tri - somies 21, 18 and 13 by cell-free DNA analysis of maternal blood at 10–11 weeks’ gestation and the combined test at 11-13 weeks. Ultrasound Obstet Gynecol, 2015, 45(1), p. 36–41.
42. Ryan, A., Baner, J., Demko, Z., et al. Informatics-based, highly accurate, noninvasive prenatal paternity testing Genet Med, 2013, 15(6), p. 473–477.
43. Salomon, LJ., Alfirevic, Z., Audibert, F., et al. ISUOG updated consensus statement on the impact of cfDNA aneuploidy testing on screening policies and pre-natal ultrasound practice. Ultrasound Obstet Gynecol, 2017, 49(6), p. 815–816.
44. Samango-Sprouse, C., Banjevic, M., Ryan, A., et al. SNP-based non-invasive prenatal testing detects sex chromosome aneuploidies with high accuracy. Prenat Diagn, 2013, 33(7), p. 643–649.
45. Sayres, LC., Cho, MK. Cell-free fetal nucleic acid testing: A review of the technology and its applications. Obstet Gynecol, 2011, 66(7), p. 431–442.
46. Emer, CS., Duque, JA., Müller, AL., et al. [Prevalence of congenital abnormalities identified in fetuses with 13, 18 and 21 chromosomal trisomy]. Rev Bras Ginecol Obstet, 2015, 37(7), p. 333–338.
47. Sekizawa, A., Kondo, T., Iwasaki, M., et al. Accuracy of fetal gender determination by analysis of DNA in maternal plasma. Clin Chem, 2001, 47(10), p. 1856–1858.
48. Sparks, AB., Struble, CA., Wang, ET., et al. Noninvasive prenatal detection and selective analysis of cell-free DNA obtained from maternal blood: evaluation for trisomy 21 and trisomy 18. Am J Obstet Gynecol, 2012, 206(4), p. 319e1–e9.
49. Tabor, A., Alfirevic, Z. Update on procedure-related risks for prenatal diagnosis techniques. Fetal Diagn Ther, 2010, 27(1), p. 1–7.
50. Tong, YK., Lo, YM. Diagnostic developments involving cell-free (circulating) nucleic acids. Clin Chim Acta, 2006, 363(1–2), p. 187–196.
51. Tsui, NB., Chim, SS., Chiu, RW., et al. Systematic micro-array based identification of placental mRNA in maternal plasma: towards non-invasive prenatal gene expression profiling. J Med Genet, 2004, 41(6), p. 461–467.
52. Van der Schoot, CE., Soussan, AA., Koelewijn, J., et al. Non-invasive antenatal RHD typing. Transfus Clin Biol, 2006, 13(1-2), p. 53–57.
53. Wald, NJ., Densem, JW., George, L., et al. Prenatal screening for Down´s syndrome using inhibin-A as a serum marker. Prenat Diagn, 1996, 16(2), p. 143–152.
54. Wataganara, T., LeShane, ES., Farina, A., et al. Maternal serum cell-free fetal DNA levels are increased in cases of trisomy 13 but not trisomy 18. Hum Genet, 2003, 112(2), p. 204–208.
55. Wellesley, D., Dolk, H., Boyd, PA., et al. Rare chromosome abnormalities, prevalence and prenatal diagnosis rates from population-based congenital anomaly registers in Europe. Eur J Hum Genet, 2012, 20(5), p. 521–526.
56. Wright, CF., Burton, H. The use of cell-free fetal nucleic acids in maternal blood for non-invasive prenatal diagnosis. Hum Reprod Update, 2009, 15(1), p. 139–151.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicineArticle was published in
Czech Gynaecology

2020 Issue 1
Most read in this issue
- Thrombotic microangiopathy and pregnancy
- Lactobacillus crispatus dominant vaginal microbita in pregnancy
- Noninvasive prenatal testing: benefits and limitations of the available tests
- Can we prevent ovarian cancer?
