Effect of systemic enzymotherapy on Cesarean section scar healing
Vplyv systémovej enzýmovej terapie na hojenie jazvy po cisárskom reze
Ciele:
Cieľom našej štúdie bolo sledovať zmeny v hojení uterotomických jaziev po cisárskom reze u pacientiek užívajúcich systémovú enzymoterapiu v porovnaní s pacientkami, ktoré neužívali systémovú enzymoterapiu (Wobenzym).
Metodika:
Prospektívna kohortová štúdia bola vykonaná u 60 prvorodičiek, ktoré rodili cisárskym rezom. Porovnávali sme nasledovné premenné: hrúbku jazvy po cisárskom reze, koeficient dehiscencie (DRC), závažnosť defektu jazvy po cisárskom reze, dilatáciu dutiny maternice, pooperačnú bolesť, C-reaktívny proteín a telesnú teplotu pacientiek.
Výsledky:
Hrúbka jazvy 6 týždňov po cisárskom reze bola významne väčšia v skupine pacientiek, ktoré dostávali Wobenzym (7,1 ± 0,9 mm; priemer ± SD), ako u pacientiek bez Wobenzymu (5,3 ± 0,7 mm) (p = 0,01). Ťažký defekt jazvy po cisárskom reze bol pozorovaný u jednej užívateľky Wobenzymu (1/30, 3,3 %) a u 5 žien (5/30, 16,7 %), ktoré Wobenzym neužívali, a teda nešlo o štatisticky významný rozdiel (p = 0,195).
Záver:
Napriek tomu, že v skupine pacientiek užívajúcich Wobenzym bolo menej ťažkých defektov jaziev po cisárskom reze, rozdiel nedosiahol štatistickú významnosť, čo možno pripísať malému súboru pacientiek.
Kľúčové slová:
enzymoterapia, cisársky rez, ultrazvuk, jazva po cisárskom reze
Authors:
Erik Dosedla 1
; A. Grendelova 1; P. Calda 2
Authors place of work:
Department of Obstetrics and Gynecology, P. J. Safarik University, 1st Private Hospital Košice-Šaca Inc., Košice-Šaca, Slovak Republic
1; Charles University, Prague, First Faculty of Medicine and General Teaching Hospital, Department of Gynecology and Obstetrics, Prague, Czech Republic
2
Published in the journal:
Ceska Gynekol 2016; 81(3): 202-207
Summary
Objectives:
The aim of our study was to monitor changes in the healing of Caesarean section scars in patients using systemic enzymotherapy in comparison with patients not treated with systemic enzymotherapy (Wobenzym).
Methods:
A prospective cohort study was conducted in 60 primiparous women delivered by CS. We compared the following outcomes: scar thickness after the Caesarean section, dehiscence risk coefficient (DRC), severity of the Caesarean section scar defect, uterine cavity dilation, post-operative pain, C-reactive protein level and febrility.
Results:
The scar thickness 6 weeks after CS was significantly greater in the group of patients taking Wobenzym (7.1±0.9 mm; mean ± SD) than in the patients without Wobenzym (5.3±0.7 mm) (p = 0.01). Severe Caesarean section scar defects were observed in 1/30 (3.3%) Wobenzym users and in 5/30 (16.7%) patients who did not use Wobenzym, with no statistically significant difference (p = 0.195).
Conclusion:
Despite the percentage of patients with a severe CS scar defect being apparently lower in the group treated with Wobenzym, the difference did not reach statistical significance due to the small size of the study population.
Keywords:
enzymotherapy, Caesarean section, ultrasound, Cesarean section scar
INTRODUCTION
With the currently increasing frequency of Caesarean sections, many more complications occur that were rare in the past. Apart from the increased risk of post-delivery bleeding, the main issue is abnormal healing of the Caesarean section scar [1]. Abnormal healing of the Caesarean section scar can lead to unpleasant clinical consequences. Firstly, the risks of uterine rupture, ectopic gravidity in the Caesarean section scar and placentation disturbances are increased in subsequent gravidities [2]. Secondly, the Caesarean section scar defect may be associated with chronic dysmenorrhoea, post-menstrual spotting, chronic pelvic pain and infertility [3, 4]. The frequency of these pathological and often life-threatening conditions varies between 0.04–3.8% [5].
Systemic enzymotherapy (SET) has long been considered to be a purely empirical therapeutic method. SET is a therapeutic method based on the complex effects of a specifically combined mixture of hydrolytic enzymes (mainly proteases of plant and animal origin), in combination with rutin, which targets key physiological and pathophysiological processes [6]. The wide range of SET indications results from its main pharmacological effects, i.e. antiedematous, fibrinolytic, antiflogistic and immunomodulatory activity, as described in Basic and Applied Pharmacology [22].
Some studies have confirmed a „vehicle effect“, i.e. increased antibiotic and chemotherapeutic bioavailability in blood and tissues when co-administered with SET [7, 23, 24, 25].
The aim of our study was to monitor changes in the healing of Caesarean section scars in patients using Wobenzym (trypsin, chymotrypsin, bromelain, papain, lipase, amylase, pancreatin and rutoside; MUCOS Pharma GmbH and Co.KG) in comparison with patients not treated with Wobenzym. We compared the following outcomes: scar thickness after the Caesarean section, dehiscence risk coefficient (DRC), severity of the Caesarean section scar defect, uterine cavity dilation, post-operative pain, C-reactive protein level and febrility.
MATERIAL AND METHODS
Between September 2014 and March 2015, 60 primiparous women participated in this prospective cohort study.
Inclusion criteria included: 1. singleton pregnancy, 2. one-layer suture of the uterotomy wound, 3. all Caesarean sections performed by one surgeon, 4. all ultrasound measurements done by one surgeon and 5. elective Caesarean section. No patients with any uterine incision or insulin-dependent diabetes mellitus were included in the study.
Participation in the Wobenzym study (first group of patients) was offered to 84 pregnant women between September 2014 and March 2015; 54 of them refused to participate. The first group included 30 pregnant women who started to use systemic enzymes (Wobenzym tablets) 12 hours after elective Caesarean section. Participation in the control group (no treatment) was offered to 92 pregnant women between December 2014 and March 2015; 62 of them refused. The patients in the first group used Wobenzym orally for 21 days, 10 tablets twice daily in a fasted state at least 30 minutes before a meal, because mixing the enzymes with food in the stomach decreases their absorption. We evaluated the following outcomes in each group: age of the mother, gestational age at the time of delivery, birth weight, uterine cavity dilation 48 and 96 hours after the SC, SC scar 6 weeks after the surgery, dehiscence risk coefficient (DRC) 6 weeks after surgery, CRP 96 hours after SC, maximum body temperature during hospitalization and pain evaluated using a visual analogue scale during hospitalization. The patients were given the same standard analgesic combination of mitamizol and tramadol after delivery (saline 500 ml + mitamizol 3 g + tramadol 400 mg in a dose of 30 ml/hour).
All pregnant women provided signed informed consent to participate in the study before the elective Caesarean section. This study was approved by the ethics committee of the Nemocnice Košice Šaca, Inc..
CAESAREAN SECTION
Patients who indicated to have their pregnancy terminated by Caesarean section received standard intravenous antibiotic prophylaxis consisting of 2 g of cefazolin. The Caesarean section was performed using a modified Misgav-Ladach method with minimal use of instruments and blunt separation of abdominal wall layers (Joel-Cohen laparotomy) [8]. With this method, the uterus is cut open above the uterovesical fold and the incision is digitally extended to the sides. Uterotonics (oxytocin 5 IU) was administered as an intravenous bolus before manual removal of the placenta. The uterine incision was sutured with a single layer of running absorbable sutures (Vicryl Plus). Abdominal cavity toilet was performed using a suction unit. The visceral peritoneum and parietal peritoneum were approximated to each other, not sutured. The rectus aponeurosis was sutured with a simple running suture using the same suture material. A Foley catheter was inserted into the urinary bladder for 24 hours after surgery. All elective Caesarean sections were made by an experienced surgeon before the start of regular contractions.
ULTRASOUND EXAMINATION
An ultrasound examination took place 48 and 96 hours (transabdominal) and 6 weeks (transvaginal) after the Caesarean section in all 60 patients. All examinations were performed by a single operator (E.D.) with a Voluson E6 BT13 ultrasound machine and a convex abdominal 4-8 MHz transducer (RAB 4-8L) and a transvaginal 5-9 MHz transducer (RIC 5-9). The physician performing the examination was not informed whether the patient was using the systemic enzymotherapy or not.
The maximum uterine cavity dilation was measured 48 and 96 hours after the Caesarean section. The patients were asked to urinate before the ultrasound examination and they were in a supine position during the examination. The probe was placed on the abdomen perpendicularly to the longitudinal axis of the uterus with only slight pressure applied to the fundus. Low ultrasound frequency (4 MHz) was used to view the entire uterus. The image was enlarged, so that the uterus with the adjacent urinary bladder and pouch of Douglas took up the entire screen. The cavity was virtual in the first week after delivery. The decidua appeared as a thin light line ranging from the fundus to the internal os of the cervix. Sometimes, this line was irregular and thicker. The material inside the uterine cavity was of various echogenicities and consisted mainly of liquid blood, blood clots and necrotic remnants of the placenta.
The ultrasound examinations of the uterine scar at week 6 were performed transvaginally. The thickness of the Caesarean section scar and the myometrium proximal and distal to the CS scar were evaluated. In order to define the severity of the scar, we introduced a dehiscence risk coefficient (DRC), which was calculated as the ratio between the thickness of the scar (s) and the thickness of the myometrium adjacent to the defect (mean thickness of the myometrium proximal (pm) and distal (dm) the scar):DRC = s/(pm+dm)×0.5.
A DRC of the scar after CS less than 0.25 was considered a severe defect based on our results in a previous study [9].
STATISTICAL ANALYSIS
PASW Statistics 18 (SPSS, Chicago, IL, USA) was used for the statistical analysis. Descriptive statistics are presented by median or mean values with standard deviation (SD) and variance, respectively. Categorical variables are expressed as absolute numbers and percentages. Non-parametric Mann-Whitney tests were used to determine the statistical differences of particular clinical outcomes. Fisher’s exact test was used to assess the relationship between Wobenzym use and the presence of a severe scar defect after CS. P-values < 0.05 were considered statistically significant.
RESULTS
After Caesarean section, all 60 women who had agreed to participate in the study underwent all study phases and three consecutive ultrasound examinations. The median age of the women in labour was 30 years (range 20–39). The mean birth weight was 3296 g (±468 g) and the median gestational age was 39 weeks (36–41). The difference in the median age of the women in labour using Wobenzym and that in the control group (no Wobenzym) was not statistically significant (p = 0.94). Accordingly no significant differences were observed in the mean birth weight (p = 0.64) or in the gestational age (p = 0.58).
The clinical and ultrasound factors evaluated at predefined time-points (48 h, 96 h, 6 weeks after the SC) are shown in table 1. The scar thickness was significantly greater in the group of patients taking Wobenzym than in the patients without Wobenzym (p = 0.01) (tab. 2). The involution of the uterine cavity was found to be significantly more rapid in the group of patients taking Wobenzym (p < 0.001) at both time-points, according to the statistical analysis of ultrasound measurements of the uterine cavity made at 48 hours and 96 hours after SC. The DRC after SC was significantly higher in the group of patients treated with Wobenzym (p = 0.008).
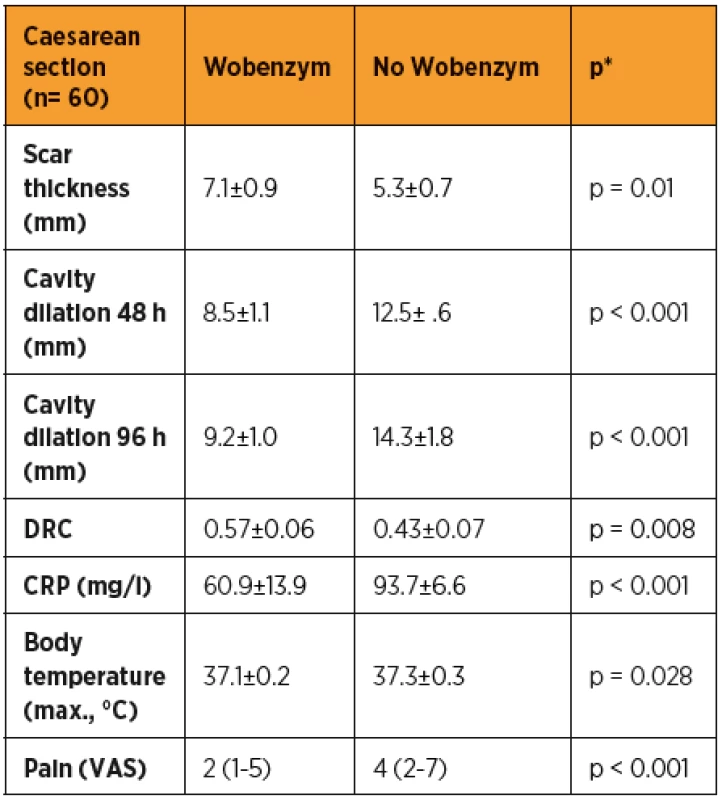
The body temperature was significantly lower in the group of patients taking Wobenzym (p = 0.02). Similarly, C-reactive protein (CRP) was significantly lower in the group of patients treated with Wobenzym (p < 0.001). The assessment of post-operative pain showed that patients treated with Wobenzym reported significantly less pain (p < 0.001) on the visual analogue scale (VAS).
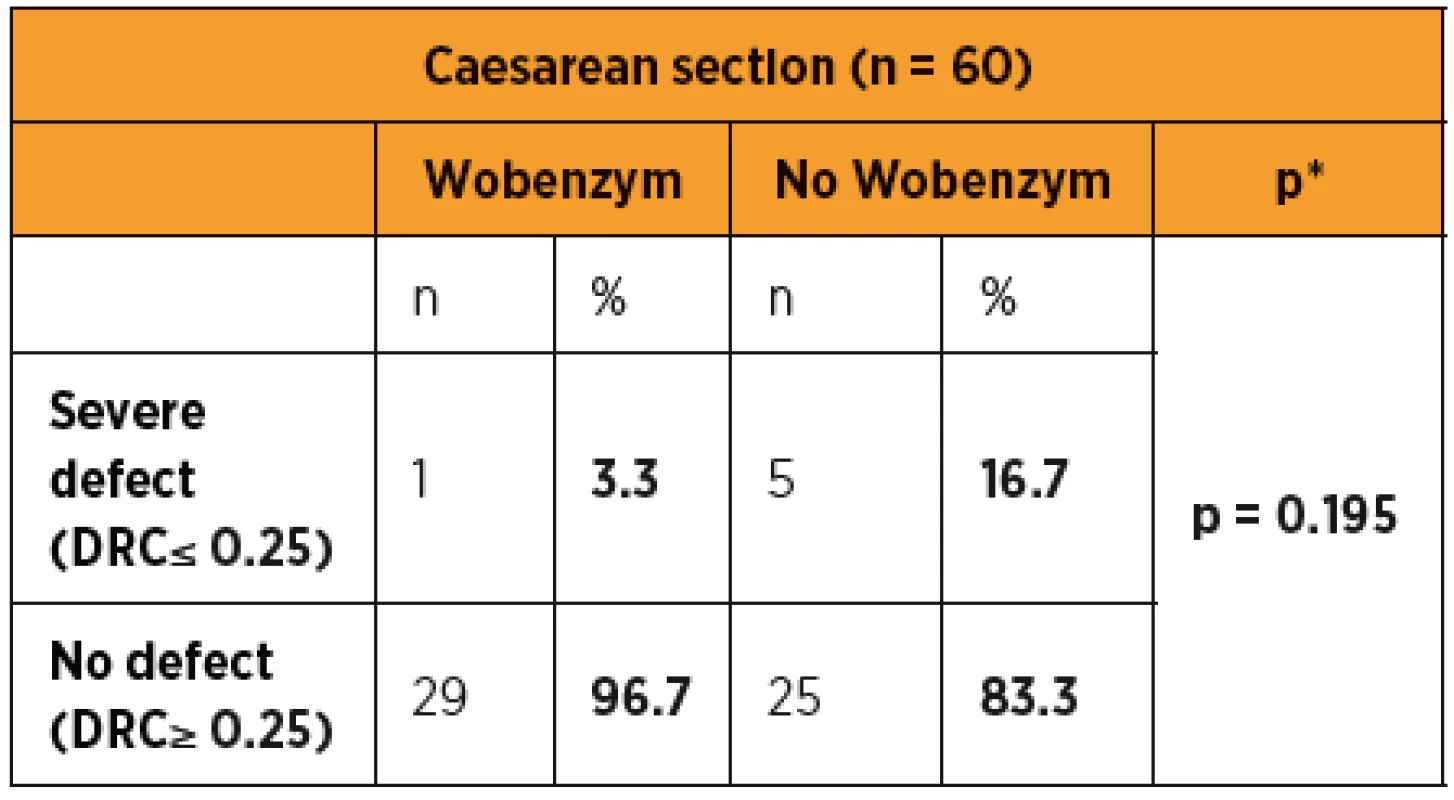
Severe Caesarean section scar defects were observed in 1/30 (3.3%) Wobenzym users and in 5/30 (16.7%) patients who did not use Wobenzym, with no statistically significant difference (p = 0.195). The statistical evaluation was affected by the small number of patients.
DISCUSSION
The statistical evaluation of outcomes indicating a successful healing process in patients after CS showed a statistically significant difference in favour of the group treated with Wobenzym in comparison to the control group for nearly all of the study outcomes (scar thickness, uterine cavity dilation after 48 and 96 hours, dehiscence risk coefficient, CRP, body temperature and pain assessment using VAS). Despite the fact that the percentage of patients with a severe defect was apparently lower in the group treated with Wobenzym, statistical significance was not reached due to the small size of the study population. If we had included patients after acute CS and in particular patients after two or more Caesarean sections in the study, we may have observed more significant differences between Wobenzym users and women who did not use Wobenzym [10].
Uterine cavity dilation was lower both 48 and 96 hours after CS in patients taking Wobenzym, which could be explained by the proteolytic effect of the enzymes contained in Wobenzym, and thus more rapid colliquation of the uterine cavity contents and consequent faster cavity restitution into the antepartum condition.
Inflammatory complications that manifested in immunocompetent patients as increased body temperature and higher CRP levels were significantly more frequent in patients not using Wobenzym. Numerous studies have shown that Wobenzym has antiedematous, anti-inflammatory and fibrinolytic effects, and that its enzymes act as antioxidants [7, 11, 17, 26]. On the other hand, doubts remain regarding the effectiveness of oral enzymotherapy. Proteolytic enzymes are large protein molecules that must be absorbed in an active form from the gastrointestinal tract [18], and very little is known about the mode of action of systemic enzymotherapy. The enzymes are produced in an enterosolvent form so as to not be destroyed in the stomach by hydrolysis. Critics of oral enzymotherapy reproach some clinical data for not being adequately controlled and being based on subjective observations [12]. There are several hypotheses on the anti-inflammatory effects of systemic enzymotherapy. Talaieva and Bratus reported their opinion that systemic enzymes act indirectly by reducing active oxygen radicals together with the antioxidant effects of their other components, rutoside and flavonoids [13]. Other authors have suggested that proteases form complexes with cytokines through specific interactions with anti-proteases freely circulating in the blood that are subsequently subject to endocytosis or phagocytosis, which increases their clearance and keeps them away from sites of inflammation [19, 20].
Bromelain, one of the components of SET, has anti-inflammatory effect by means of attenuating of the effects of IL-1, IL-6 and TNF-α released from THP-1 cells14. Braun et al. in their study administered bromelain or a placebo to children with acute sinusitis. The symptoms were relieved significantly more rapidly in children using bromelain in comparison with the group of children treated with standard therapy only [15]. The anti-inflammatory and immunomodulatory effects of systemic enzymotherapy have also been shown in Czech studies. For example, Wobenzym had demonstrated effects on reducing the number of inflammation events and associated antibiotic use in children with recurrent respiratory infections [21].
Uterotomy wound healing is influenced by many factors. The most important include retroflexed uterus, acute Caesarean section and iterative Caesarean sections [10]. We also assessed post-operative pain using VAS in our study. The patients who used Wobenzym reported significantly less pain. This was also found in a double-blind study by Bolten et al. They compared pain and knee-joint function in patients with osteoarthritis treated with Wobenzym (three tablets twice daily), the non-steroidal anti-inflammatory drug diclofenac (150 mg daily) or placebo for 12 weeks. The Wobenzym benefit was comparable to diclofenac and significantly more pronounced in comparison to the group of patients with placebo. Adverse effects were similar in patients using Wobenzym and placebo [16].
We are aware of the limitations of this study, in particular the lack of a placebo control, masking and the small number of patients. The findings, patient compliance and tolerability of Wobenzym were very good. The majority of the objectively measured outcomes showed a statistically significant difference in comparison to the control group. The patients from our population who used Wobenzym had significantly lower mean body temperature and C-reactive protein levels after elective Caesarean section, which are very good indicators of a lower inflammatory burden on the body, as well as lower uterine cavity dilation (better retraction) and less pain, as well as significantly better healing of the Caesarean section scar, i.e. greater scar thickness and DRC.
Despite the percentage of patients with a severe defect being apparently lower in the group treated with Wobenzym, the difference did not reach statistical significance due to the small size of the study population.
It is possible to conclude that both subjective and objective benefits from the oral enzymotherapy were observed in the early post-operative period.
Condensation
A severe CS scar defect being apparently lower in the group treated with Wobenzym, the difference did not reach statistical significance.
Acknowledgements
Supported by the project of the Ministry of Health, Czech Republic, for conceptual development of research organization MZ ČR – RVO64165, General University Hospital in Prague, Czech Republic.
Erik Dosedla, MD
Department of Obstetrics and Gynecology
P. J. Safarik University,1st Private Hospital Košice-Šaca Inc.
Košice-Šaca, Slovak Republic
e-mail: edosedla@gmail.com
Zdroje
1. Dosedla, E., Calda, P. Ultrazvuková diagnostika maternice v šestonedelí. Actual Gyn, 2011, 3, p. 52–60.
2. Fitzpatrick, KE., Kurinczuk, JJ., Alfirevic, Z., et al. Uterine rupture by intended mode of delivery in the UK: a national case-control study. PLoS Med, 2012, 9(3), p. e1001184. Epub 2012 Mar 13.
3. Dosedla, E. Ultrazvuková diagnostika jaziev na maternici po cisárskom reze. Moderní gynekologie a porodnictví. 2012, 21, 4, s. 490-497.
4. Wang, CB., Chiu, WW., Leem, CY., et al. Cesarean scar defect: correlation between Cesarean section number. Defect size, clinical symptoms and uterine position. Ultrasound Obstet Gynecol, 2009, 34(1), p. 85–89.
5. Bij de Vaate, AJ., Brölmann, HA., van der Voet, LF., et al. Ultrasound evaluation of the Cesarean scar: relation between a niche and postmenstrual spotting. Ultrasound Obstet Gynecol, 2011, 37(1), p. 93–99.
6. Paradise, ME., Couture, P., Gigleux, I., et al. Impact of systemic enzyme supplementation on low-grade inflammation in human. Pharma Nutrition. 2015 Article in Press.
7. Klein, G., Kullich, W., Schnitker, J., Schwann, H. Efficacy and tolerance of an oral enzyme combination in painful osteoarthritis of the hip. A double-blind. randomised study comparing oral enzymes with non-steroidal anti-inflammatory drugs. Clin Exp Rheumatol, 2006, 24, p. 25–30.
8. Goodlin, RC. Modified Joel-Cohen technique for Caesarean delivery. Brit J Obstet Gynaecol, 1999, 106(12), p. 1329.
9. Dosedla, E., Calda, P., Kvasnička, T. Ultrasonography of the uterus within 6 weeks following Cesarean section. Cent Eur J Med, 2012, 7(2), p. 235–240.
10. Ofili-Yebovi, D., Ben-Nagi, J., Sawyer, E., et al. Deficient lower-segment Cesarean section scars: prevalence and risk factors. Ultrasound Obstet Gynecol, 2008, 31, p. 72.
11. Wittenborg, A., Bock, PR., Hanisch, J., et al. Comparative epidemiological study in patients with rheumatic diseases illustrated in an example of a treatment with non-steroidal anti-inflammatory drugs versus an oral enzyme combination preparation] Arzneimittelforschung, 2000, 50, p. 728–738.
12. Shah, SA., Nerurkar, RP. Evaluation of prescribing trends and rationality of use of oral proteolytic enzymes. Indian J Pharmacol, 2013, 45(3), p. 309–310.
13. Talaieva, TV., Bratus, VV. Proteolytic enzyme combination reduces inflammation and oxidative stress and improves insulin sensitivity in a model of metabolic syndrome. Advances in Enzyme Res, 2015, 3(1), p. 1–8.
14. Huang, JR., Wu, CC., Hou, RC., Jeng, KC. Bromelain inhibits lipopolysaccharide-induced cytokine production in human THP-1 monocytes via the removal of CD14. Immunol Invest, 2008, 37, p. 263–277.
15. Braun, JM., Schneider, B., Beuth, HJ. Therapeutic use, efficiency and safety of the proteolytic pineapple enzyme Bromelain-POS in children with acute sinusitis in Germany. In Vivo, 2005, 19, p. 417–421.
16. Bolten, WW., Glade, MJ., Raum, S., Ritz, BW. The safety and efficiency combination in managing knee osteoarthritis pain in adults: a randomized, double blind, placebo-controlled trial. Arthritis, 2015.
17. Neumayer, C., Fugl, A., Nanobashvili, J., et al. Combine enzymatic and antioxidative treatment reduces ischemia-reperfusion injury in rabbit skeletal muscle. J Surg Res, 2006, 133(2), p. 150–158.
18. Lorkowski, G. Gastrointestinal absorption and biological activities of serine and cysteine proteases of animal and plantorigin: review on absorption of serine and cysteine proteases. Int J Physiol Pathophysiol Pharmacol, 2012, 4(1), p. 10–27.
19. Desser, L., Holomanova, D., Zavadova, E., et al. Oral therapy with proteolytic enzymes decreases excessiveTGF-beta levels in human blood. Cancer Chemother Pharmacol, 2001, 47, Suppl, p. S10–S15.
20. Lauer, D., Müller, R., Cott, C., et al. Modulation of growth factor binding properties of alpha2-macroglobulin by enzymetherapy. Cancer Chemother Pharmacol, 2001, 47, Suppl. p. S4–S9.
21. Adámková, E., Balcar, J., Bartovičová, E., et al. Systémová enzymoterapie v komplexní léčbě recidivujících zánětů dýchacích cest u dětí – postregistrační retrospektivní multicentrické hodnocení. Čes.-slov. Pediatrie, 2004, 59(1), s. 513–521.
22. Jezdínský, J. Systémová enzymoterapie. In: Lincová, D., Farghali, H., eds. Základní a aplikovaná farmakologie. 2. vydání. Praha: Galén, 2007, s. 606–611.
23. Luerti, M., Vignali, M. Influence of bromelain on penetration of antibiotics in uterus, salpinx and ovary. Drugsexptl Clin Res, 1978, 4(1), p. 45–48.
24. Sukhikh, GT., Loginova, NS., Faizullin, LZ., et al. The use of Wobenzym® to facilitate interferon synthesis in the treatment of chronic urogenital chlamydiosis. Int J Immunotherapy, 1997, 3/4, p. 131–133.
25. Förstl, M., Kalousek, I., Navrátil, P., et al. Zkušenosti s enzymoterapií v rámci komplexní léčby urogenitálních infekcí Chlamydia trachomatis. Urolog pro Praxi, 2006, 5, p. 243–245.
26. Rahn, HD. Begleitende Therapie durch hydrolytische Enzyme bei arthroskopischer Meniskusresektion. Prakt Sport-Traumatol Sportmedizin,1994, 10(3), p. 123–127.
Štítky
Dětská gynekologie Gynekologie a porodnictví Reprodukční medicínaČlánek vyšel v časopise
Česká gynekologie

2016 Číslo 3
Nejčtenější v tomto čísle
- Traumatic symphyseal rupture by vaginal delivery,report of a rare case
- The possibility of antepartal prevention of episiotomy and perineal tears during delivery
- Spontaneus delivery after two previous caesarean sections – case report
- Surgical treatment of endometriomas and ovarian reserve
