Once daily oral GnRH antagonist and add-back therapy – successful medical treatment of heavy menstrual bleeding due to leiomyoma and adenomyosis causing severe anaemia
Authors:
J. Racková 1,2
Authors‘ workplace:
Department of Obstetrics and Gynaecology, University Hospital Bulovka, Prague, Czech Republic
1; Gynem s. r. o., Prague, Czech Republic
2
Published in:
Ceska Gynekol 2023; 88(2): 135-138
Category:
Commercially Supported Communication
Introduction
Uterine benign myometrial disorders, such as leiomyoma (L) and/or adenomyosis (A), play a pivotal role in many aspects of women’s reproductive life. They contribute to a major cause of hypermenorrhoea, polymenorrhoea, abnormal uterine bleeding, and increased risk of anaemia. Symptomatic patients experience decreased quality of life, altered sexual well-being, dysmenorrhea, pelvic pain, infertility, pregnancy complications, as well as an increased risk of pregnancy loss. Leiomyomas are one of the most common female diseases. They are benign monoclonal neoplasms that affect women mainly during their reproductive years and are diagnosed in up to 70–80% of women during their lifetime [1]. There is a role of ovarian steroid hormones plateau in the pathogenesis of uterine fibroids. Estrogens (E) and progesterone (PR) induce cells to release mitogenic stimuli. PR action is required for the complete development and proliferation of the cells. Estrogens predominantly increases tissue sensitivity to PR by increasing the availability of PR receptors [2]. Women, regardless of fibroid location, are equally likely to report heavy bleeding or be diagnosed with anaemia. However, among women with complaints of pelvic pain or heavy bleeding, uterine weight was found to be higher in women with at least one submucosal leiomyoma [3].
Adenomyosis is defined by the presence of islands of endometrial glands and stroma surrounded by hypertrophic smooth muscle cells within the myometrium and was initially described by Rokitansky in 1860. E is an important factor for adenomyosis growth and development. It stimulates endothelial progenitor cells and de novo formation of capillaries. The increased concentration of E and estrogen receptors (ER) stimulates ectopic presence of endometrial tissue composed of glands and stroma and affects peristalsis of uterine smooth muscle, causing pain, menometrorrhagia, infertility, and adverse obstetric outcomes [4].
Both L/A commonly coexist, sometimes with endometriosis showing a myriad of symptoms. Concomitant adenomyosis in hysterectomy specimens of women with leiomyomas ranges from 15 to 57% [5]. In vitro data suggest that L/A share some common pathogenetic mechanisms. Specific cytogenetic rearrangements including deletion of chromosome 7q and dysregulation of the fibroblast growth factor (FGF) system have been reported in both conditions [6].
Treatment options
Several therapeutic modalities are available; asymptomatic cases can be managed expectantly with close monitoring of the progression. Symptomatic uterine fibroids require adequate therapy according to the severity of symptoms, age, infertility, request to preserve the uterus and FIGO classification.
Surgical therapy
Hysterectomy still accounts for almost three-quarters of all surgical therapies. Other therapeutic modalities include hysteroscopy with transcervical resection, minimally invasive surgery, laparoscopic myomectomy, minilaparotomy or laparotomy depending on the myoma size and location. However, when a myometrial suture is needed, there is an increased risk of uterine rupture and abnormal placentation during pregnancy or delivery. Surgical therapy of adenomyosis remains difficult and the techniques are sporadic.
Radiologic therapy
The alternative to surgical treatment of leiomyoma can be minivasive percutaneous interventions – uterine fibroid embolization, ablation or focused ultrasound, interstitial laser thermoablation or cryomyolysis. They can be preferred in women who desire to preserve their uterus.
Medical therapy
Medical treatment aims on suppressing ovulation and menstrual bleeding and can be used only in women with no contraindication for hormonal therapy. Hormonal contraceptives and Gestagens can be used to improve heavy menstrual bleeding associated with fibroids, primarily through their suppressive effects on endometrial proliferation, but overall they do not affect decreasing uterine fibroid volume or uterine size. Other therapeutic modalities include the levonorgestrel-releasing intrauterine system 53 mg LNG-IUS [7].
Injectable GnRH receptor agonists can be used to clinically treat abnormal uterine bleeding associated with uterine leiomyoma.
Relugolix is an orally available GnRH antagonist that binds and blocks the receptor in the anterior pituitary gland, prevents GnRH binding to the receptor, and inhibits the subsequent release of LH and FSH. Relugolix add-back therapy (RAB) consists of 40 mg relugolix, 1 mg estradiol, and 0.5 mg norethisterone acetate. It was developed as a once-daily oral treatment to achieve efficacy and minimize vasomotor symptoms and bone mineral density loss by maintaining estradiol concentrations within a therapeutic range consistent with those in the early follicular phase of the menstrual cycle.
In the LIBERTY placebo-controlled trials, once-daily RAB reduced menstrual blood loss volume and pain in women with heavy menstrual bleeding associated with uterine leiomyomas and was well tolerated, with preservation of bone mineral density compared to placebo. SPIRIT study assessed the effect of RAB on pain reduction in patients with surgically diagnosed endometriosis. Dysmenorrhea and moderate to severe pelvic pain also significantly improved compared to the placebo. During the clinical trials, complete amenorrhea was achieved in about 50% of patients, and heavy menstrual bleeding was improved in 73% of women [8,9].
Case report
A 47-year-old female, was referred to our clinic from the Department of Haematology for severe sideropenic anaemia related to chronic abnormal uterine bleeding. Her haemoglobin level was initially 67 g/L. She suffered from chronic hyper-/poly-menorrhea. She had to use the extra-large hygienic pads 20 times a day, and she suffered from worsening dizziness in the past 6 months. At first, she was treated by her general practitioner with 320 mg ferrous sulphate and 60 mg ascorbic acid orally, and then she consulted a haematologist. The blood count results revealed severe hypochromic microcytic anaemia, reticulosis, reactive thrombocytosis, and decreased ferritin levels. Tranexamic acid (500 mg, two tablets) three times a day at the start of menstrual bleeding did not have a major effect neither on the improvement of the symptoms nor on the laboratory results.
Clinical history revealed catamenial migraines non-reactive to standard analgesics therapy in the past 2 years; she used Eletriptan with a reasonable effect. Fifteen years ago she underwent laparoscopy for infertility, chronic pelvic pain, peritoneal endometriosis, deep endometriosis of the vesical plica and urinary bladder were found. Hysteroscopy did not show any pathologies. She went through several cycles of assisted reproduction, she suffered one miscarriage and had 2 successful pregnancies. She delivered by caesarean section. In the past two years, she suffered from worsening dysmenorrhea, pelvic pain, and paraesthesia of the left lower limb. No severe pathologies were found on the conciliary sonography at that time. She was concerned about her altered and decreased sexual life, and abused different analgesics. She refused any hormonal treatments for some time. Her mammogram did not show any risk of malignancies.
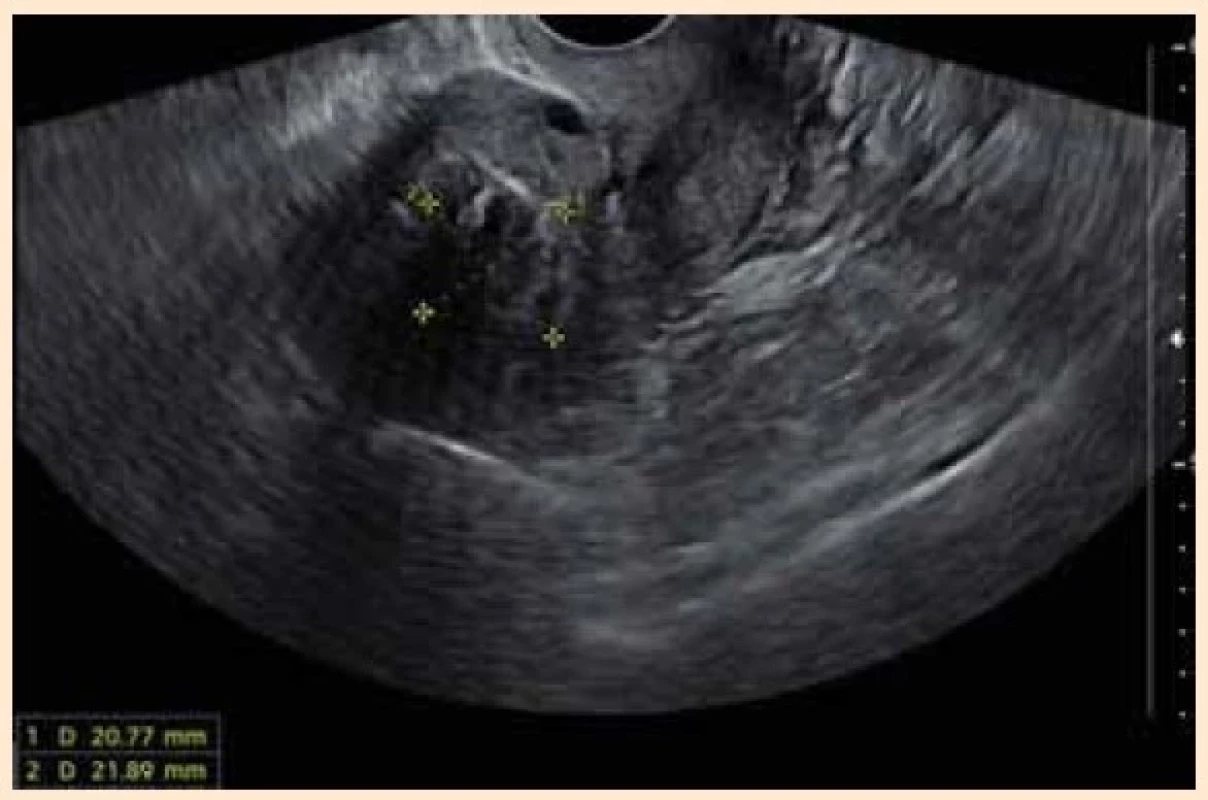
A sonographic exam revealed 32 × × 30 mm transmural submucosal leiomyoma and two foci of focal adenomyosis 9 × 9 mm in the anterior and posterior uterine wall (Fig. 1). The patient was not willing to undergo any surgical treatment or hysterectomy.
Obr. 1. Sagitální sonografické zobrazení dělohy s transmurálním
submukózním myomem před nasazením terapie.

The patient finally agreed on the first-line treatment with oral contraceptives and Gestagens. However, the 3-month treatment was unsuccessful and did not improve any of the symptoms. The patient signed the informed consent with the publication of her case.
Major outcomes of the treatment with Relugolix and add-back therapy:
- the patient became asymptomatic during the first month of treatment resulting in complete amenorrhea;
- the levels of haemoglobin and thrombocytes normalized within three months of treatment Graph 1;
- uterine size and uterine leiomyoma size decreased, and endometrium size was low (Fig. 2);
- improved quality of life, better sexual well-being;
- no further dysmenorrhea. No catamenial migraines;
- decreased use of analgesics and antimigraine drugs, no major side effects;
- no major side effects after one year of treatment.
Graf 1. Postupná elevace hodnoty hemoglobinu do fyziologického rozmezí.

Obr. 2. Sagitální sonografické zobrazení dělohy se
zmenšenou velikostí myomu po terapii Relugolixem
s kombinovanou terapií.
Discussion
As shown in large multinational clinical trials, RAB is safe in treating heavy menstrual bleeding, improve abdominal pain, and dysmenorrhea related to leiomyoma.
In the past decades, women required alternatives to surgical treatment to maintain fertility and preserve reproductive functions. They may be anxious about being away from daily activities and work; there are those who already had several abdominal operations. Some women also have a higher risk of leiomyoma recurrence, pelvic pain, and bleeding after surgery. Risks and benefits of hysterectomy with ovarian preservation at any age are presented in the article by Laughlin-Tommaso from the Mayo Clinic [10].
Hysterectomy can shorten the time to menopause by 2–4 years and increase long-term risks of cardiovascular disease, in particular in women who undergo a hysterectomy at a young age. There is an increased risk of urinary incontinence in some individuals after surgery [11].
Severe anaemia from heavy menstrual bleeding deserves high attention and a multidisciplinary approach. RAB can decrease or stop uterine bleeding and can offer an alternative approach to surgical intervention. An individualized approach and tailored therapy are needed due to the heterogeneity of the symptoms and clinical findings in patients with L/A. If indicated, clinicians can encourage women to try the treatment for 3–6 months and extend it more if the goals of the treatment are met and are well tolerated. Patient should be advised about a regular follow-up to assess the effect of therapy. As seen in our case of severe sideropenic anaemia, the patient suffered dizziness and migraines related to her menstrual cycle and used antifibrinolytics, antimigraine agents and analgesics. She did not use them during treatment with RAB. Adequate iron supplementation should also be recommended. Relugolix has a half-life of 37–42 hours; however, if there is low compliance, the risk of recurrence of symptoms and pregnancy may increase. Breast cancer screening and densitometry can be recommended in perimenopausal women or women at risk. Patients should be advised about all the therapeutic options regarding L/A. A novel once daily oral GnRH antagonist with combination therapy is a promising and modern type of treatment designed to improve the symptoms in women who want to preserve their uterus.
Jana Racková, MD
Department of Obstetrics and Gynaecology
University Hospital Bulovka
Budínova 67/2
181 00 Praha 8
Czech Republic
Podpořeno společností Gedeon Richter Marketing ČR, s. r. o.
Sources
1. Giuliani E, As-Sanie S, Marsh EE. Epidemiology and management of uterine fibroids. Int J Gynaecol Obstet 2020; 149 (1): 3–9. doi: 10.1002/ijgo.13102.
2. Reis FM, Bloise E, Ortiga-Carvalho TM. Hormones and pathogenesis of uterine fibroids. Best Pract Res Clin Obstet Gynaecol 2016; 34: 13–24. doi: 10.1016/j.bpobgyn.2015.11.015.
3. Bachmann GA, Bahouth LA, Amalraj P et al. Uterine fibroids: correlations of anemia and pain to fibroid location and uterine weight. J Reprod Med 2011; 56 (11–12): 463–466.
4. Crha K, Ješeta M, Pilka R et al. Adenomyosis – its possible effect on endometrial function and receptivity. Ceska Gynekol 2021; 86 (3): 205–209. doi: 10.48095/cccg2021205.
5. Weiss G, Maseelall P, Schott LL et al. Adenomyosis a variant, not a disease? Evidence from hysterectomized menopausal women in the Study of Women’s Health Across the Nation (SWAN). Fertil Steril 2009; 91 (1): 201–206. doi: 10.1016/ j.fertnstert.2007.11.025.
6. Taran FA, Weaver AL, Coddington CC et al. Understanding adenomyosis: a case control study. Fertil Steril 2010; 94 (4): 1223–1228. doi: 10.1016/j.fertnstert.2009.06.049.
7. Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update 2016; 22 (6): 665–686. doi: 10.1093/humupd/dmw023.
8. Stewart EA, Lukes AS, Venturella R et al. Relugolix combination therapy for uterine leiomyoma-associated pain in the LIBERTY randomized trials. Obstet Gynecol 2022; 139 (6): 1070–1081. doi: 10.1097/AOG.0000000000004 787.
9. Giudice LC, As-Sanie S, Arjona Ferreira JC et al. Once daily oral relugolix combination therapy versus placebo in patients with endometriosis-associated pain: two replicate phase 3, randomised, double-blind, studies (SPIRIT 1 and 2). Lancet 2022; 399 (10343): 2267–2279. doi: 10.1016/S0140-6736 (22) 00622-5.
10. Laughlin-Tommaso SK, Stewart EA. Moving toward individualized medicine for uterine leiomyomas. Obstet Gynecol 2018; 132 (4): 961–971. doi: 10.1097/AOG.0000000000002 785.
11. Altman D, Granath F, Cnattingius S et al. Hysterectomy and risk of stress-urinary-incontinence surgery: nationwide cohort study. Lancet 2007; 370 (9597): 1494–1499. doi: 10.1016/S01 40-6736 (07) 61635-3.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicineArticle was published in
Czech Gynaecology

2023 Issue 2
Most read in this issue
- Anti-Müllerian hormone – clinical use and future possibilities
- Adnexal torsion in childhood and adolescence
- Specifics of infertility treatment in women with premature ovarian failure
- Severe hepatic rupture in HELLP syndrome
