Use of serum copper and zinc levels in the diagnostic evaluation of endometrioma and epithelial ovarian carcinoma
Využití sérových hladin mědi a zinku při diagnostickém hodnocení endometriomu a epiteliálního karcinomu vaječníků
Cíl: Cílem této studie je zhodnotit sérové hladiny mědi (Cu) a zinku (Zn) u pacientek s epiteliálním karcinomem ovaria a endometriomem. Materiál a metody: Do studie bylo zařazeno 21 pacientek s epiteliálním karcinomem ovaria, 47 pacientek s endometriomem, 31 zdravých žen v reprodukčním věku a 10 zdravých žen v menopauze. Byly porovnány hladiny Cu a Zn a poměry Cu/Zn. Výsledky: Ve skupině s endometriomem byly hladiny Cu (p = 0,04) a poměr Cu/Zn (p < 0,01) vyšší, zatímco hladiny Zn (p < 0,01) byly nižší ve srovnání s kontrolní skupinou. Prahová hodnota 1,15 s 62% senzitivitou a 61% specificitou byla vypočtena pro poměr Cu/Zn pomocí ROC křivky (AUC = 0,688; p = 0,005). Ve skupině s rakovinou vaječníků byly hladiny Cu (p ≤ 0,01) a poměr Cu/Zn (p = 0,02) vyšší, zatímco hladiny Zn (p ≤ 0,02) byly nižší ve srovnání s kontrolní skupinou. Prahová hodnota poměru Cu/Zn 1,37 byla vypočtena se 76% senzitivitou a 90% specificitou (AUC = 0,829; p = 0,004). Hladina Zn byla nižší (p = 0,02) a poměr Cu/Zn byl vyšší (p = 0,01) ve skupině s karcinomem ovaria ve srovnání se skupinou s endometriomem. Závěr: Prahová hodnota poměru Cu/Zn pro karcinom ovaria mohla být stanovena se specificitou 90 %, zatímco senzitivita a specificita poměru Cu/Zn pro endometriom byly nízké.
Klíčová slova:
karcinom vaječníků – měď – zinek – endometriom – poměr měď zinek
Authors:
Z. E. Utkan Korun 1
; M. Erdem 2; A. Erdem 2; A. Onan 2
; N. Bozkurt 2
; M. Öktem 2
; K. Biberoğlu 2
Authors‘ workplace:
Department of Obstetrics and Gynecology, Acıbadem Maslak Hospital, Istanbul, Turkey
1; Department of Obstetrics and Gynecology, Gazi University School of Medicine, Ankara, Turkey
2
Published in:
Ceska Gynekol 2023; 88(4): 279-286
Category:
Original Article
doi:
https://doi.org/10.48095/cccg2023279
Overview
Objective: The aim of this study is to evaluate serum copper (Cu) and zinc (Zn) levels in patients with epithelial ovarian cancer and endometrioma. Materials and methods: We included 21 epithelial ovarian cancer patients, 47 endometrioma patients, 31 healthy women of reproductive age, and 10 healthy women in menopause. Cu and Zn levels and Cu/Zn ratios were compared. Results: In the endometrioma group, Cu levels (P = 0.04) and Cu/Zn ratio (P < 0.01) were higher, while Zn levels (P < 0.01) were lower compared to the control group. The threshold value of 1.15 with 62% sensitivity and 61% specificity was calculated for the Cu/Zn ratio using the ROC curve (AUC = 0.688; P = 0.005). In the ovarian cancer group, Cu levels (P ≤ 0.01) and Cu/Zn ratio (P = 0.02) were higher, whereas Zn levels (P ≤ 0.02) were lower compared to the control group. The Cu/Zn ratio threshold value of 1.37 was calculated with 76% sensitivity and 90% specificity (AUC = 0.829; P = 0.004). The Zn level was lower (P = 0.02), and the Cu/Zn ratio was higher (P = 0.01) in the ovarian cancer group compared to the endometrioma group. Conclusion: The threshold value of the Cu/Zn ratio for ovarian cancer could be determined with a specificity of 90%, whereas the sensitivity and specificity of the Cu/Zn ratio for endometrioma were low.
Keywords:
endometrioma – ovarian cancer – Zinc – copper – copper zinc ratio
Introduction
Endometriosis is a progressive estrogen-dependent inflammatory disease associated with pelvic pain and infertility, with a risk of malignant transformation [1]. Ovarian endometriosis is one of the most common causes of adnexal masses in reproductive age. Although endometriotic lesions are mostly benign, it is known that endometriomas increase the risk of endometroid and clear cell carcinoma [2]. Research continues for reliable and non-invasive methods that can be used in the differential diag- nosis of malignancy when evaluating adnexal masses.
One of the proposed mechanisms of malignant transformation is through reactive oxygen species (ROS). ROS produced by endometrial cells scattered in the peritoneal cavity can lead to DNA damage and somatic mutations [3]. Trace elements are structural compounds of most enzymes involved in ROS production and inhibition. Among them, copper (Cu) and zinc (Zn) are widely involved in the cell’s oxidant and antioxidant processes [4,5]. An increase in serum Cu concentration and a parallel decrease in serum Zn levels have been demonstrated in numerous pathological conditions [6,7]. In addition, several studies in the literature show that the serum Cu/Zn ratio is significantly increased in patients with endometriosis and those with cervical, ovarian and endometrial cancer [8–12].
The aim of this study is to determine whether serum Cu Zn levels and Cu/Zn ratio differ in patients diagnosed with endometrioma or ovarian cancer compared to the healthy population. We also aimed to compare the Cu/Zn ratios between the endometrioma and ovarian cancer groups to define possible threshold values that can be used in the differential diagnosis.
Materials and methods
Study Population
The present study included 21 patients diagnosed with epithelial ovarian cancer and 47 patients diagnosed with endometrioma between February 2014 and August 2014 in Gazi University Hospital, Ankara. Thirty-one healthy women of reproductive age and 10 healthy women in menopause were included in the control group. Exclusion criteria were pregnancy, breastfeeding, comorbidities, history of hormonal therapy for endometriosis within three months, history of medications containing Cu or Zn within three months, adnexal mass other than endometrioma, previous surgery for ovarian cancer or other malignancies, and history of chemotherapy or radiation. Also, patients with any gynecological or non-gynecological pathologies detected during the pelvic examination and ultrasonographic evaluation were excluded from the control groups. Additional information was obtained regarding the age at menarche, menstrual cycle duration, gravidity, parity, and contraceptive use. A regular menstrual cycle was defined as an interval of 25–35 days pre-cycle.
Dysmenorrhea, dyspareunia, and pelvic pain severity of the patients were scored according to the Biberoğlu & Behrman scale [13].
This prospective study is approved by the Gazi University Hospital Institutional Ethics Committee for the use and analysis of patient information and data.
Biochemical Assay
Serum Cu, Zn and Ca 125 levels were analyzed in blood samples of 5 cc collected from the median cubital vein of enrolled women into vacutainers treated with ethylenediaminetetraacetic acid anticoagulant following 10–12 hours fasting. The samples were centrifuged at 1,000 g for 5 minutes. Separated serum samples were diluted 1/10 using deionized water. Subsequently, serum Cu and Zn levels were analyzed using an Atomic Absorption Spectrometer (AAS, Shimadzu/AAS 7000) [14,15]. Analytical wavelength was 324.8 nm for Cu and 213.9 nm for Zn and slit width was 0.7 nm. Measurement of each element was performed in triplicate and the average pf the measurement values was taken into account. Cu and Zn concentrations were given as micrograms per deciliter (mcg/dL). Cu/Zn ratios were calculated by direct division of these values. The reference ranges for serum Cu are as follows: 84–155 mcg/dL. The reference range for serum Zn is 78–157 mcg/dL. Serum Ca 125 level was obtained as a tumor marker for malignancy screening. Serum Ca 125 levels were analyzed by the ECLIA (Electrochemiluminescence Immunoassay) method using an autoanalyzer (Roche Diagnostics Ltd., Switzerland) in blood samples taken in SST tubes [16]. Correlation between serum Ca 125 level and Cu/Zn ratio were also checked in the study groups. All samples were analyzed in the Gazi University Faculty of Medicine Medical Biochemistry Department Laboratory.
Analysis
Statistical analyses were performed with SPSS version 20.0 (IBM, Armonk, NY, USA). Continuous data were reported as mean ± Standard Deviation (SD). The normality of the distributions of the variables was confirmed by the Shapiro--Wilk test. Parametric tests were used for numerical variables with normal distribution, and nonparametric tests were used for numerical variables that did not show normal distribution. Comparisons between groups were made using a Student’s T-test and Mann-Whitney U-Test. The predictive accuracies of statistically significant variables were evaluated using an ROC analysis. The relationships of the variables with each other were determined with a Pearson’s correlation. P-value of less than 0.05 was considered to be statistically significant.
Results
A total of 109 participants, 21 patients with epithelial ovarian cancer and 47 patients with endometrioma, were included in the study. The control group for endometrioma comprised of 31 healthy women of reproductive age, and the control group for ovarian cancer consisted of 10 healthy women in menopause without any pathology detected in the pelvic examination and ultrasound evaluation. Characteristics of patients are summarized in Tab. 1.
Tab. 1. Charakteristika pacienta.

*comparison between endometrioma vs. reproductive control groups
**comparison between ovarian cancer vs. menopause control groups
Comparison of endometrioma vs reproductive control group
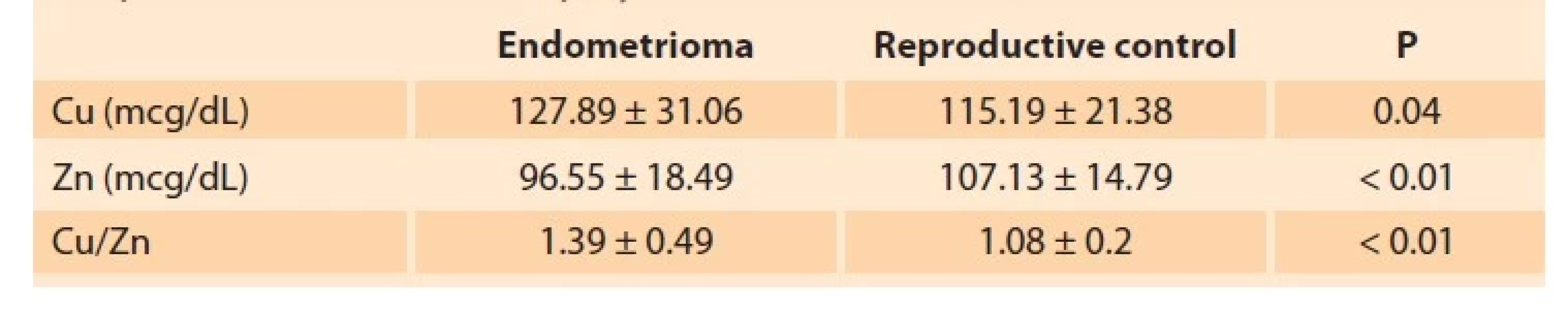
The mean age of 47 patients in the endometrioma group was 32.5 ± 6.17 (18–45) years. The mean age of 31 women in the reproductive control group was 35.9 ± 8.07 (19–45) years. There was no significant difference between the two groups regarding age (P = 0.053) and menarche age (P = 0.06). In the reproductive control group, 25 patients were fertile. In the endometrioma group, 19 were infertile, and 34 patients (72%) had a histopathological diagnosis. Sixteen patients were operated on a pelvic mass, 16 for infertility, and two for pelvic pain. Of these, 13 patients (38%) were classified as stage 3 endometriosis, whereas 21 patients (62%) were stage 4. The distribution of patients in the endometrioma group according to the severity of symptoms is shown in Fig. 1. In the endometrioma group, the Cu level was 127.89 ± 31.06 mcg/dL, the Zn level was 96.55 ± 18.49 mcg/dL, and the Cu/Zn ratio was 1.39 ± 0.49. In the reproductive control group, the Cu level was 115.19 ± 21.38 mcg/dL, the Zn level was 107.13 ± 14.79 mcg/dL, and the Cu/Zn ratio was 1.08 ± 0.2. In the endometrioma group, Cu levels (P = 0.04) and Cu/Zn ratio (P < 0.01) were significantly higher, while Zn levels (P < 0.01) were significantly lower. Cu levels were negatively correlated with dyspareunia (Pearson correlation: –0.35; P = 0.03). There was no other correlation between Cu and Zn levels and the stage of endometriosis or symptoms of the disease. Also, no significant correlation was found between serum Cu or Zn levels and Ca 125 values. Sensitivity and specificity studies of various threshold rates for the Cu/Zn ratio were conducted to evaluate the diagnostic value of the elements. The threshold value of 1.15 with 62% sensitivity and 61% specificity was calculated for the Cu/Zn ratio using the ROC curve (AUC = 0.69; P < 0.01: 0.005) (Fig. 2). Cu levels, Zn levels, and Cu/Zn ratio determined in endometrioma and reproductive control group are given in Tab. 2.
Obr. 1. Rozdělení pacientů podle závažnosti symptomů ve skupině endometriomů.

Obr. 2. ROC křivka pro poměr Cu/Zn pro endometriom AUC = 0,688; p = 0,005.
Prahová hodnota 1,15 s 62% senzitivitou a 61% specificitou.

Tab. 2. Hladina mědi (Cu), hladiny zinku (Zn) a poměr Cu/Zn u endometriomu
a reprodukční kontrolní skupiny.
Comparison of ovarian cancer vs menopause control group
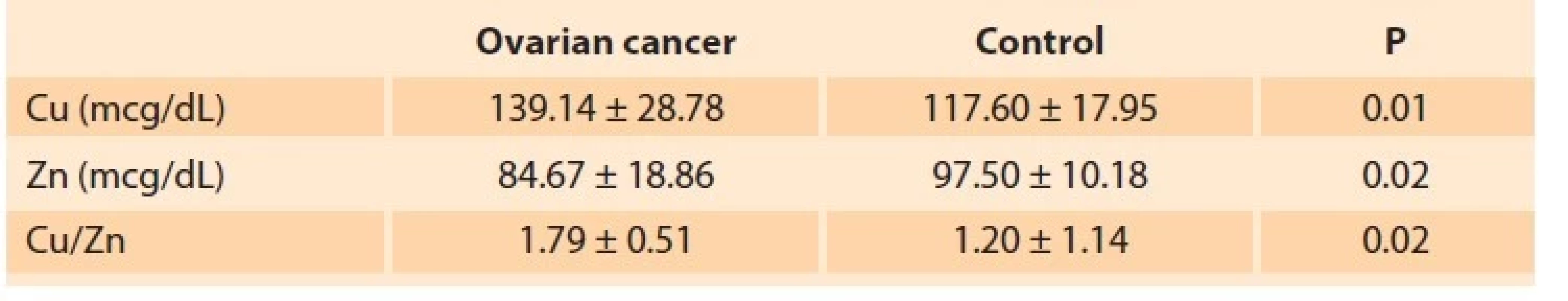
The mean age of 21 patients in the ovarian cancer group was 54.33 ± 13.53 (30–77) years. The mean age of 10 menopausal women in the control group was 57.40 ± 6.28 (50–68) years. There was no significant difference between the two groups regarding age (P = 0.28). According to the FIGO (the International Federation of Gynecology and Obstetrics) staging system for ovarian cancer, four patients were stage 1, 13 were stage 3, and four were stage 4. Cu levels, Zn levels, and Cu/Zn ratio determined in ovarian cancer and menopause control group are given in Tab. 3. In the ovarian cancer group, Cu levels (P < 0.01) and Cu/Zn ratio (P = 0.02) were significantly higher, and Zn levels (P = 0.02) were significantly lower. There was no correlation between Cu/Zn ratio and ovarian cancer stage or Ca 125 levels. The sensitivity and specificity of different threshold values were determined with the ROC curve to evaluate the diagnostic value of the Cu/Zn ratio in ovarian cancer. A cut-off value of 1.37 was calculated with 76% sensitivity and 90% specificity (AUC = 0.83; P < 0.01) (Fig. 3).
Tab. 3. Hladina mědi (Cu), hladiny zinku (Zn) a poměr Cu/Zn u rakoviny
vaječníků a kontrolní skupiny.
Obr. 3. ROC křivka pro poměr Cu/Zn pro karcinom vaječníků AUC = 0,829; p = 0,004.
Prahová hodnota 1,37 se 76% senzitivitou a 90% specificitou.

Comparison of ovarian cancer group and endometrioma group
In the ovarian cancer group, the Zn level was significantly lower (P = 0.02), and the Cu/Zn ratio was significantly higher (P = 0.01). Although the Cu level was higher in ovarian cancer, this difference was not statistically significant (P = 0.16).
Cross-comparison of the control groups
Cu levels, Zn levels, and Cu/Zn ratios were compared between endometrioma vs. menopause control groups, ovarian cancer vs. reproductive control groups and menopause control vs. reproductive control groups, respectively. Only the comparison between ovarian cancer and reproductive control groups yielded significant differences. In the ovarian cancer group, Cu level (P = 0.02) and Cu/Zn ratio (P < 0.01) were significantly higher, and Zn level (P < 0.01) was significantly lower compared to the reproductive control group. The relationship of Cu/Zn ratios between all four groups is demonstrated in Fig. 4.
Obr. 4. Je ukázán vztah poměrů Cu/Zn mezi všemi čtyřmi skupinami. Skupiny byly
seřazeny v sestupném pořadí na ose X s ohledem na poměry Cu/Zn. Průměrné
poměry Cu/Zn u ovariálního karcinomu, endometriomu, menopauzální kontroly
a reprodukční kontrolní skupiny byly 1,79 ± 0,51; 1,39 ± 0,49; 1,20 ± 1,14;
1,08 ± 0,2; v tomto pořadí.

Discussion
The need for non-invasive methods to be used in the differential diagnosis of endometrioma and ovarian cancer remains current. Transvaginal ultrasonography is often sufficient for the diagnosis of endometriomas [17]. However, it may be challenging to distinguish endometriomas from malignant tumors due to high serum Ca-125 levels or the ultrasonographic appearance of nonhomogeneous cyst content, or wall irregularities caused by hemosiderin deposition [18]. According to a previous study, using only ultrasonography, 0.2–0.9% of malignancies can be misclassified as endometriomas in premenopausal women [19]. Ca-125 is the most commonly used marker to detect ovarian cancer in patients with an adnexal mass. However, the sensitivity and specificity of Ca-125 are low, and many gynecological conditions, such as endometriosis, may lead to elevated levels of Ca-125, especially in premenopausal women [20]. Therefore, various molecules, including trace elements that are considered to have a role in the pathogenesis of endometriosis, are being investigated as diagnostic markers.
Cu is a redox-active trace element, and both its deficiency and excess are associated with an increase in oxidative stress. On the other hand, Zn is a trace element accepted as an antagonist of redox-active metals due to its antioxidant and anti-inflammatory properties [6]. In the present study, it was found that serum Cu levels were significantly higher and serum Zn levels were significantly lower in both the endometrioma and ovarian cancer groups compared with healthy control groups. Limited data evaluates the relationship between endometrioma and trace elements in the literature. Turgut et al showed that serum Cu levels and oxidative stress markers were significantly higher in patients with stage 3 and 4 endometriosis than in the control group [12]. However, serum Zn levels were not evaluated in this study. Recently, blood levels of various trace elements, including zinc and copper, were determined in Asian patients with and without endometriosis. It was reported that blood zinc levels were significantly lower in women with endometriosis, whereas blood levels of copper were not significantly different between the two groups [21]. The present study is one of the few in the literature evaluating serum Cu and Zn levels together in endometrioma patients, and our results are in line with previous reports.
The results of the studies evaluating trace elements in ovarian cancer vary in the literature. Lightman et al compared the serum Cu and Zn levels of patients with benign and malignant ovarian tumors and found that serum Cu levels were significantly higher. In contrast, serum Zn levels were significantly lower in the malignant group [22]. Jafari Shobeiri et al compared the serum Cu and Zn levels in patients with malignant and benign ovarian tumors. They reported that serum Cu levels increased significantly in the malignant group, while the decrease in serum Zn levels was not statistically significant [10]. In another study evaluating serum Cu and Zn levels in patients with cervical, endometrial, and ovarian cancer, no significant difference was found in trace element levels in patients with ovarian cancer. However, 16 ovarian cancer patients were included in the study, and the stages of these patients were not specified [23]. A recent meta-analysis including twenty case-control studies comparing serum Cu and Zn levels between patients with ovarian cancer and benign ovarian lesions reported that circulating Cu concentrations were significantly higher and circulating Zn concentrations were significantly lower in ovarian cancer patients. The authors also remarked that a suggestive causal association was only detected with Zn concentration, suggesting further studies on Zn interventions for ovarian cancer might have a clinical impact [8]. The above-mentioned studies included patients with various adnexal masses as control groups. It has not been clearly reported whether there is endometrioma among them. Marinov et al analyzed serum Cu and Zn levels in patients with benign ovarian cysts, endometrioma, and ovarian cancer along with the healthy population. Serum Cu levels were highest in the ovarian cancer group, followed by patients with benign ovarian cysts and endometrioma, respectively [24]. The authors found no difference in Zn levels in the three patient groups. However, five patients were included in the endometrioma group and six in the ovarian cancer group. In the present study, patients with adnexal masses other than endometrioma and ovarian cancer were not included. Our results showed that the serum Zn level was significantly lower in the ovarian cancer group than in the endometrioma group. The Cu level tended to be higher in the ovarian cancer group, but the difference was not statistically significant. The use of Cu and Zn levels as a diagnostic test in ovarian cancer should be confirmed with a larger series.
Cu/Zn ratios have been evaluated in various studies for differential diagnosis. This study compared the Cu/Zn ratios among all groups. The ratio was significantly higher in patients with ovarian cancer than in the endometrioma group. Also, the ratio was higher in the endometrioma group compared to the healthy population. These findings are comparable with the results of other studies in the literature. The Cu/Zn ratio was found to be significantly higher in patients with ovarian cancer than in the group with benign ovarian tumors [10,22,25]. It has also been reported that the Cu/Zn ratio was positively correlated with the ovarian cancer stage [22]. In the study of Marinov et al the Cu/Zn ratio was found to be the highest in the ovarian cancer group, respectively, followed by the benign ovarian tumor group and then the endometrioma group [24].
In this study, the sensitivity and specificity of various threshold values for the Cu/Zn ratio to be used in the diagnosis of ovarian cancer and endometrioma were studied. The threshold value of 1.37 was calculated for the Cu/Zn ratio in the ovarian cancer group, with a sensitivity of 76% and a specificity of 90%. Lightman et al reported a threshold value of 1.87 for malignant ovarian tumors and emphasized that values between 1.65–1.87 should be evaluated carefully regarding malignancy [22]. Jafari Shobeiri et al calculated the threshold value for malignant ovarian tumors as 2.04, with a sensitivity of 73% and a specificity of 70% [10]. Differences in these results may be due to the number of patients, difficulties in measuring trace elements, their interactions with each other, and the fluctuation of their levels in the body. In this study, the threshold value for endometrioma was calculated as 1.14, with a sensitivity of 62% and a specificity of 61%. To the best of our knowledge, there is no calculated threshold value for endometrioma in the literature.
A primary limitation of this study is that the control group for endometrioma consisted of patients who applied to our outpatient clinic for a routine check-up. Although the gynecological examination was normal, there was no histopathological confirmation for the lack of endometriosis in these patients. Another limitation is that some of the patients in the endometrioma group did not have a histopathologic diagnosis.
Recently, new screening tests and iindices, such as RMI or ROMA, that use several markers together to predict the malignancy potential of ovarian lesions have been generated [26]. Due to the limited variables of our study population, we were not able to compare our results with markers other than Ca-125, which can be considered another limitation. Also, one other limitation of this study is that the small number of patients causes difficulty in interpreting the data. Further studies with a larger number of patients should be conducted to clarify the predictive value of Cu and Zn.
Conclusions
A reliable and effective marker to differentiate malignant and benign masses is needed. This study is one of the few in the literature evaluating both serum Cu and Zn levels and a threshold value of Cu/Zn ratio together in endometrioma patients. The ratio was highest in the ovarian cancer group and lowest in the healthy group. Although the threshold value for differentiating ovarian cancer from healthy controls was calculated with a specificity of 90%, the sensitivity and specificity of the Cu/Zn ratio for differentiating endometrioma was low. Therefore, the Cu/Zn ratio is of limited value for differential diagnosis. The use of Cu and Zn levels as a diagnostic test in ovarian cancer should be confirmed with a larger series.
Authorship
ZEUK: conceptualization, resources, writing – original draft, writing review and editing
ME: methodolgy, resources, formal analysis, writing – original draft
AE: resources, writing – original draft
AO: resources, writin – original draft
NB: resources, writing – original draft
MÖ: resources, writing – original draft
KB: conceptualization, methodology, formal analysis, resources, conceptualization, writing – original draft, supervision
ORCID authors
Z. E. Utkan Korun 0000-0002-1595-569X
A. Onan 0000-0001-7643-1585
N. Bozkurt 0000-0002-1107-9629
M. Öktem 0000-0002-5555-8189
K. Biberoğlu 0000-0003-3236-8742
Submitted/Doručeno: 7. 3. 2023
Accepted/Přijato: 9. 5. 2023
Zeynep Ece Utkan Korun, MD
Department of Obstetrics and Gynecology
Acıbadem Maslak Hospital
Büyükdere Cd. No: 40
34398 Sarıyer/Istanbul
Turkey
Sources
1. Bulun SE, Yilmaz BD, Sison C et al. Endometriosis. Endocr Rev 2019; 40 (4): 1048–1079. doi: 10.1210/er.2018-00242.
2. Samartzis EP, Labidi-Galy SI, Moschetta M et al. Endometriosis – associated ovarian carcinomas: insights into pathogenesis, diagnostics, and therapeutic targets – a narrative review. Ann Transl Med 2020; 8 (24): 1712. doi: 10.21037/atm-20-3022a.
3. Kobayashi H. Potential scenarios leading to ovarian cancer arising from endometriosis. Redox Rep 206; 21 (3): 119–126. doi: 10.1179/1351000215Y.0000000038.
4. Gupte A, Mumper RJ. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat Rev 2009; 35 (1): 32–46. doi: 10.1016/j.ctrv.2008.07.004.
5. Yaman M, Kaya G, Simsek M. Comparison of trace element concentrations in cancerous and noncancerous human endometrial and ovary tissues. Int J Gynecol Cancer2007; 17 (1): 220–228. doi: 10.1111/j.1525-1438.2006.00742.x.
6. Michalczyk K, Cymbaluk-Płoska A. The role of zinc and copper in gynecological malignancies. Nutrients 2020; 12 (12): 3732. doi: 10.3390/nu12123732.
7. Kazi Tani LS, Gourlan AT, Dennouni-Medjati N et al. Copper isotopes and copper to zinc ratio as possible biomarkers for thyroid cancer. Front Med (Lausanne) 2021; 8: 698167. doi: 10.3389/fmed.2021.698167.
8. Lin S, Yang H. Ovarian cancer risk according to circulating zinc and copper concentrations: a meta-analysis and Mendelian randomization study. Clin Nutr 2021; 40 (4): 2464–2468. doi: 10.1016/j.clnu.2020.10.011.
9. Cunzhi H, Jiexian J, Xianwen Z et al. Serum and tissue levels of six trace elements and copper/zinc ratio in patients with cervical cancer and uterine myoma. Biol Trace Elem Res 2003; 94 (2): 113–122. doi: 10.1385/BTER: 94: 2: 113.
10. Shobeiri MJ, Tabrizi AD, Atashkhoei S et al. Serum levels of copper, zinc and copper/zinc ratio in patients with ovarian cancer. Pakistan J Med Sci 2011; 27 (3): 561–565.
11. Atakul T, Altinkaya SO, Abas BI et al. Serum copper and zinc levels in patients with endometrial cancer. Biol Trace Elem Res 2020; 195 (1): 46–54. doi: 10.1007/s12011-019-01844-x.
12. Turgut A, Özler A, Görük NY et al. Copper, ceruloplasmin and oxidative stress in patients with advanced-stage endometriosis. Eur Rev Med Pharmacol Sci 2013; 17 (11): 1472–1478.
13. Biberoglu KO, Behrman SJ. Dosage aspects of danazol therapy in endometriosis: short- -term and long-term effectiveness. Am J Obstet Gynecol 1981; 139 (6): 645–654. doi: 10.1016/ 0002-9378 (81) 90478-6.
14. Elmer P, Conn N. Analytical methods for atomic absorption spectrophotometery. London 1975.
15. Application news A634. Direct analysis of metallic elements in cell culture medium by atomic absorption spectrophotometry (AAS). Shimadzu 2020.
16. Hasanbegovic L, Alicelebic S, Sljivo N. Comparison of specific ovarian tumor markers by elecsys analyzer 2010. Acta Inform Med 2015; 23 (2): 86–89. doi: 10.5455/aim.2015.23.86-89.
17. Becker CM, Bokor A, Heikinheimo O et al. ESHRE guideline: endometriosis. Hum Reprod Open 2022; 2022 (2): hoac009. doi: 10.1093/ hropen/hoac009.
18. Testa AC, Timmerman D, van Holsbeke C et al. Ovarian cancer arising in endometrioid cysts: ultrasound findings. Ultrasound Obstet Gynecol 2011; 38 (1): 99–106. doi: 10.1002/uog.8970.
19. van Holsbeke C, van Calster B, Guerriero S et al. Endometriomas: their ultrasound characteristics. Ultrasound Obstet Gynecol 2010; 35 (6): 730–740. doi: 10.1002/uog.7668.
20. Králíčková M, Vetvicka V, Fiala L et al. The search for biomarkers in endometriosis: a long and windy road. Reprod Sci 2022; 29 (6): 1667–1673. doi: 10.1007/s43032-021-00668-2.
21. Lai GL, Yeh CC, Yeh CY et al. Decreased zinc and increased lead blood levels are associated with endometriosis in Asian Women. Reprod Toxicol 2017; 74: 77–84. doi: 10.1016/j.reprotox.2017. 09.001.
22. Lightman A, Brandes JM, Binur N et al. Use of the serum copper/zinc ratio in the differential diagnosis of ovarian malignancy. Clin Chem 1986; 32 (1 Pt 1): 101–103.
23. Okonkwo CA, Amegor FO, Gbolade JO. Relationship between trace elements and major gynaecological malignancies. Asian J Med Sci 2013; 6: 124–127. doi: 10.19026/ajms.5.5347.
24. Marinov B, Tsachev K, Doganov N et al. The copper concentration in the blood serum of women with ovarian tumors (a preliminary report). Akush Ginekol (Sofiia) 2000; 39 (2): 36–37.
25. Brandes JM, Lightman A, Druhan A et al. The diagnostic value of serum copper/zinc ratio in gynecological tumors. Acta Obstet Gynecol Scand 1983; 62 (3): 225–229. doi: 10.3109/00016348309155796.
26. Al Musalhi K, Al Kindi M, Al Aisary F et al. Evaluation of HE4, CA-125, Risk of Ovarian Malignancy Algorithm (ROMA) and Risk of Malignancy Index (RMI) in the preoperative assessment of patients with adnexal mass. Oman Med J 2016; 31 (5): 336–344. doi: 10.5001/omj.2016.68.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicineArticle was published in
Czech Gynaecology

2023 Issue 4
Most read in this issue
- The efficacy of human papillomavirus vaccination in the prevention of recurrence of severe cervical lesions
- Therapeutical strategies for recurrent endometrial cancer
- Direct abdominal muscle diastasis and stress urinary incontinence in postpartum women
- Effect of umbilical cord drainage after spontaneous delivery in the third stage of labor
