Amniotic fluid soluble CD93 is elevated in the presence of intra-amniotic inflammation in preterm prelabor rupture of the fetal membranes
Intra-amniální zánět u předčasného odtoku plodové vody před termínem porodu je spojen se zvýšením hladin sCD93 v plodové vodě
Cíl: Stanovit hladiny solubilní formy CD93 (sCD93) v plodové vodě u pacientek s předčasným odtokem plodové vody (PPROM – preterm prelabor rupture of membranes) s ohledem na přítomnost mikrobiální invaze do amniální dutiny (MIAC – microbial invasion of the amniotic cavity) a/nebo intraamniálního zánětu. Metody: Do studie bylo zahrnuto celkem144 žen s jednočetným těhotenstvím komplikovaným PPROM. Plodová voda byla získána amniocentézou. MIAC byla stanovena kultivačními a nekultivačními technikami. Intraamniální zánět byl stanoven hladinou interleukinu 6 ≥ 3 000 pg/ml v plodové vodě. Ženy byly rozděleny do následujících skupin: i) intraamniální infekce, ii) sterilní intraamniální zánět, iii) kolonizace a iv) negativní plodová voda. Hladiny sCD93 v plodové vodě byly stanoveny pomocí testu ELISA. Výsledky: Hladiny sCD93 v plodové vodě se lišily mezi skupinami žen s PPROM s intraamniální infekcí, sterilním intraamniálním zánětem, kolonizací a negativní plodovou vodou (intraamniální infekce: medián 22,3 ng/ml, sterilní intraamniální zánět: medián 21,0 ng/ml, kolonizace amniové dutiny: 8,7 ng/ml, negativní plodová voda: medián 8,7 ng/ml; p < 0,0001). Závěr: Intraamniální zánět u PPROM, bez ohledu na přítomnost, či chybení MIAC, je spojen se zvýšením hladin sCD93 v plodové vodě.
Klíčová slova:
předčasný porod – interleukin 6 – apoptóza – monocyt – neutrofil – receptor
Authors:
R. Spacek 1; M. Kacerovský 2,3
; C. Andrýs 4
; O. Souček 4
; R. Kukla 5
; R. Bolehovská 5; I. Musilová 2
Authors place of work:
Department of Obstetrics and Gynecology, Faculty of Medicine, Ostrava University, University Hospital Ostrava
1; Department of Obstetrics and Gynecology, Faculty of Medicine, Charles University, University Hospital Hradec Kralove
2; Biomedical Research Center, University Hospital Hradec Kralove
3; Department of Clinical Immunology and Allergy, Faculty of Medicine, Charles University, University Hospital Hradec Kralove
4; Institute of Clinical Biochemistry and Dia gnostics, Faculty of Medicine, Charles University, University Hospital Hradec Kralove
5
Published in the journal:
Ceska Gynekol 2022; 87(6): 388-395
Category:
Původní práce
doi:
https://doi.org/10.48095/cccg2022388
Summary
Objective: To determine the soluble form of CD93 (sCD93) concentration in amniotic fluid from pregnancies complicated by preterm prelabor rupture of membranes (PPROM) based on the presence of microbial invasion of the amniotic cavity (MIAC) and/or intra-amniotic inflammation. Methods: A total of 144 women with a singleton pregnancy complicated by PPROM were included in this study. Amniotic fluid samples were obtained by transabdominal amniocentesis. MIAC was determined by the combination of cultivation and non-cultivation techniques. Intra-amniotic inflammation was characterized as a concentration of interleukin-6 ≥ 3,000 pg/mL in amniotic fluid. Women were categorized in the following groups: i) intra-amniotic infection (both MIAC and intra-amniotic inflammation), ii) sterile intra-amniotic inflammation (intra-amniotic inflammation per se), iii) colonization of the amniotic cavity (MIAC per se), and iv) negative amniotic fluid (without both MIAC and intra-amniotic inflammation). Levels of sCD93 in amniotic fluid were assessed by ELISA. Results: A difference in the levels of sCD93 in amniotic fluid was found among the groups of women with intra-amniotic infection, sterile intra-amniotic inflammation, colonization of the amniotic cavity, and negative amniotic fluid (intra-amniotic infection: median 22.3 ng/mL, sterile intra-amniotic inflammation: median 21.0 ng/mL, colonization of the amniotic cavity: 8.7 ng/mL, negative: median 8.7 ng/mL; P < 0.0001). Conclusions: Intra-amniotic inflammation in PPROM, irrespectively of the presence or absence of MIAC, is associated with the elevation of the level of sCD93 in amniotic fluid.
Keywords:
Monocytes – Neutrophils – preterm delivery – apoptosis – interleukin-6 – receptor
Introduction
Preterm prelabor rupture of membranes (PPROM) represents a specific phenotype of spontaneous preterm delivery characterized by the rupture of fetal membranes with leakage of amniotic fluid before the onset of regular uterine activity prior to 37 weeks of gestation [1–3]. PPROM is characterized by breaching of the barrier between the intra-amniotic and vaginal/cervical environments [1–4]. Therefore, PPROM can become complicated by the ascension of bacteria from the vagina or the cervix, thus leading to the presence of microorganisms in the amniotic cavity termed microbial invasion of the amniotic cavity (MIAC) that is followed by the development of intra-amniotic inflammation [5–10].
Microorganisms in amniotic fluid are recognized by specialized pattern recognition receptors given the specific conservative motifs on their surfaces [11–16]. The activation of pattern recognition receptors triggers a well-orchestrated inflammatory response leading to the production of various inflammatory mediators [12,16–23] and to the attraction of immune cells in amniotic fluid [24–26] with an ultimate goal of solving this microbial threat.
Immune cells have on their surfaces various protein molecules, termed cluster of differentiations or classification determinant (CD), which provide targets for immunophenotyping of the immune cells [27–32]. However, an ectodomain of CD can be cleaved proteolytically from the surface of the immune cells and released into extracellular space [33,34]. The shedding of that extracellular part of the CD molecule leads to the production of a soluble form of CD (sCD) [33–35], which may have a bioactive role [35,36]. Given this, immune cells in amniotic fluid are an important source of various sCDs in amniotic fluid.
CD93 represents a transmembrane glycoprotein expressed on the surfaces of circulating myeloid cells, platelets, maturing B cells, endothelial cells, and stem cells [37–40]. CD93 is involved in the processes of the efficiency of apoptosis and the regulation of cell-cell interaction [41–48]. An extracellular part of CD93 shed from the surface of activated human monocytes and neutrophils generates sCD93 [41–48]. The function of sCD93 has yet to be fully elucidated; however, a recent study revealed that sCD93 serves as an opsonin, bridging the apoptotic cells that bind to phagocytes [37].
The change in concentrations of sCD93 in serum and plasma has been described during various inflammatory [49–51], autoimmune [52], and endocrine conditions [53]. However, there is a paucity of information as to whether sCD93 can be identified in amniotic fluid and whether its concentration in amniotic fluid reflects the presence of microorganisms in amniotic fluid and in intra-amniotic inflammation.
To answer these questions, we conducted this study to quantify the sCD93 level in amniotic fluid from PPROM pregnancies based on the presence of MIAC and/or intra-amniotic inflammation.
Materials and methods
This retrospective cohort study was carried out on pregnant women admitted to the Department of Obstetrics and Gynecology of the University Hospital Hradec Kralove (Hradec Kralove, Czech Republic) between August 2017 and January 2019. Women with a singleton pregnancy complicated by PPROM between 24+0 and 36+6 weeks of gestation were recruited. Women younger than 18 years of age and those with pregnancy-related and/or other medical complications, such as fetal growth restriction, congenital or chromosomal fetal abnormalities, gestational or pregestational diabetes, gestational hypertension, preeclampsia, signs of fetal hypoxia, or significant vaginal bleeding, were excluded from the study.
Gestational age was determined by first-trimester fetal biometry. Women with PPROM between 24+0 and 34+6 weeks of gestation were treated with antibiotics and corticosteroids (beta- methasone) to accelerate lung maturation and to reduce neonatal mortality and morbidity, whereas antibiotic treatment alone was administered to those beyond 34+6 weeks of gestation. Women with proven intra-amniotic infection (the presence of both MIAC and intra-amniotic inflammation) beyond 28 weeks of gestation were actively managed. Among the actively managed women, labor was induced or an elective cesarean section was performed within 72 hours of admission. The remaining women with PPROM were managed expectantly.
PPROM was diagnosed based on the proven pooling of amniotic fluid in the posterior fornix of the vagina or by the presence of insulin-like growth factor – binding proteins in the vaginal fluid (Actim PROM test; Medix Biochemica, Kauniainen, Finland), in the event of clinical doubt.
All women provided written informed consent prior to the collection of amniotic fluid. The collection of amniotic fluid samples for research was approved by the Institutional Review Board of the University Hospital Hradec Kralove (July 2014; Decision No. 201407 S14P), and written informed consent was obtained from each participant, all of whom were Caucasian.
Amniotic fluid sampling
Ultrasonography-guided transabdominal amniocentesis was performed at the time of admission prior to the administration of corticosteroids, antibiotics, or tocolytics. Approximately 2–3 mL of amniotic fluid were aspirated. The remaining amniotic fluid was immediately divided among four polypropylene tubes. The first tube was immediately transported to the laboratory for the amniotic fluid interleukin-6 (IL-6) assessment. The second and third tubes were transported to a microbiology laboratory for polymerase chain reaction (PCR) analysis of Ureaplasma species, Mycoplasma hominis, and Chlamydia trachomatis; PCR and sequencing of the 16S rRNA gene; and aerobic and anaerobic cultivation of cells. The fourth tube with amniotic fluid, designated for research purposes, was centrifuged for 15 min at 2.000 g to remove the cells and debris; the supernatant was divided into aliquots and stored at −80 °C until analysis.
Quantitation of sCD93 in amniotic fluid
Levels of sCD93 in amniotic fluid were assessed with enzyme-linked immunosorbent assays (ELISA) by means of the Human C1qR1/CD93 Immunoassay Kit (R&D Systems, Inc., Minneapolis, MN, USA) according to the manufacturers’ instructions, respectively. The sensitivity of the kit was 0.006 ng/mL. The absorbance values were read at 450 nm on a Multiskan RC ELISA reader (Thermo Fisher Scientific, Waltham, MA, USA). Amniotic fluid samples were 3-fold diluted.
Amniotic fluid IL-6 concentrations
IL-6 concentrations were assessed by the immuno-analyzer Cobas e602, a part of the Cobas 8000 platform (Roche Diagnostics, Basel, Switzerland). The measurement range was 1.5–50,000 pg/mL. The coefficients of inter- and intra-assay precisions were < 10% [54].
Detection of Ureaplasma species, M. hominis, and C. trachomatis
DNA was isolated from amniotic fluid using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions (using the protocol for isolating bacterial DNA from biological fluids). Real-time PCR was conducted on a Rotor-Gene 6000 instrument (Qiagen) using the commercial AmpliSens® C. trachomatis/Ureaplasma/M. hominis-FRT Kit (Federal State Institution of Science, Central Research Institute of Epidemiology, Moscow, Russia) to detect DNA from Ureaplasma species, M. hominis, and C. trachomatis in the same PCR tube (multiplex format). As a control, we included a PCR run for beta-actin, a housekeeping gene, to examine the presence of inhibitors of the polymerase chain reaction.
Detection of other bacteria in amniotic fluid
Bacterial DNA was identified by PCR that targeted the 16S rRNA gene via the utilization of primers [55,56]. Each reaction contained 3 μL of target DNA, 500 nM forward and reverse primers, and Q5 High-Fidelity DNA polymerase (NEB, Ipswich, MA, USA) in a total volume of 25 μL. The amplification was carried out on a 2720 Thermal Cycler (Applied Biosystems, Foster City, CA, USA). The products were visualized on an agarose gel. Positive reactions yielded 950 bp products that were subsequently analyzed by sequencing. The 16S rDNA PCR products were purified and subjected to sequencing with the above-named primers and the BigDye Terminator Kit v.3.1 (Thermo Fisher Scientific). The bacteria were then typed via searches for the obtained sequences in BLAST® and SepsiTestTM BLAST.
Aerobic and anaerobic cultures of amniotic fluid
The amniotic fluid samples were cultured on Columbia agar with sheep’s blood, a Gardnerella vaginalis selective medium; MacConkey agar, a Neisseria-selective medium (modified Thayer–Martin medium); Sabouraud agar; or Schaedler anaerobe agar. The plates were cultured for 6 days and checked daily. The species were identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry with the MALDI Biotyper software (Bruker Daltonics, Bremen, Germany).
Clinical diagnosis
MIAC was defined as the presence of microorganisms detected in amniotic fluid cell cultures and/or by the detection of microbial nucleic acids in amniotic fluid. Intra-amniotic inflammation was defined as a concentration of IL-6 in amniotic fluid ≥ 3,000 pg/mL. Based on the presence of MIAC and/or intra-amniotic inflammation, the women were divided into the following groups:
• intra-amniotic infection – concomitant presence of MIAC and intra-amniotic inflammation;
• sterile intra-amniotic inflammation – presence of intra-amniotic inflammation without MIAC;
• colonization of the amniotic cavity –presence of MIAC without intra-amniotic inflammation;
• negative amniotic fluid – absence of both MIAC and intra-amniotic inflammation.
Statistical analyses
The women’s demographic and clinical characteristics were compared by using the nonparametric Kruskal-Wallis test for continuous variables and were presented as a median value (IQR – interquartile range). Categorical variables were compared by the χ2 2 test and were presented as a number (%). The normality of the data was tested via the D’Agostino-Pearson omnibus normality test and the Shapiro-Wilk test. Because the amniotic fluid CD93 levels were not normally distributed, the nonparametric Kruskal-Wallis and Mann-Whitney U tests were performed for statistical analyses, and the results were presented as a median value (IQR). Spearman’s partial correlation analysis was performed to adjust the results for gestational age at sampling, maternal age, and smoking. Differences were considered significant at P < 0.05. All P-values were obtained by two-tailed tests, and all statistical analyses were performed in GraphPad Prism, version 8.1.1. for Mac OS X (GraphPad Software, San Diego, CA, USA) or in the Statistical Package for Social Sciences (SPSS), version 19.0 for Mac OS X (SPSS Inc., Chicago, IL, USA).
Results
In total, 144 women were included in the study. The presence of MIAC and IAI was observed in 25% (36/144) and 21% (30/144) of the women, respectively. The women’s demographic and clinical data based on the presence or absence of MIAC are shown in Tab. 1. The microorganisms identified in the samples of amniotic fluid were as follows: Ureaplasma spp. (N = 23), Haemophilus influenzae (N = 4), Streptococcus spp. (N = 2), Lactobacillus crispatus (N = 2), Mycoplasma hominis (N = 1), Chlamydia trachomatis (N = 1), Ureaplasma spp. + Mycoplasma hominis (N = 1), and Ureaplasma spp. + Gardnerella vaginalis (N = 1).
Tab. 1. Demografická a klinická charakteristika žen s předčasným odtokem plodové vody před termínem porodu s ohledem
na přítomnost intra-amniální infekce, sterilního intra-amniálního zánětu, kolonizace do amniální dutiny a negativní
plodovou vodu.
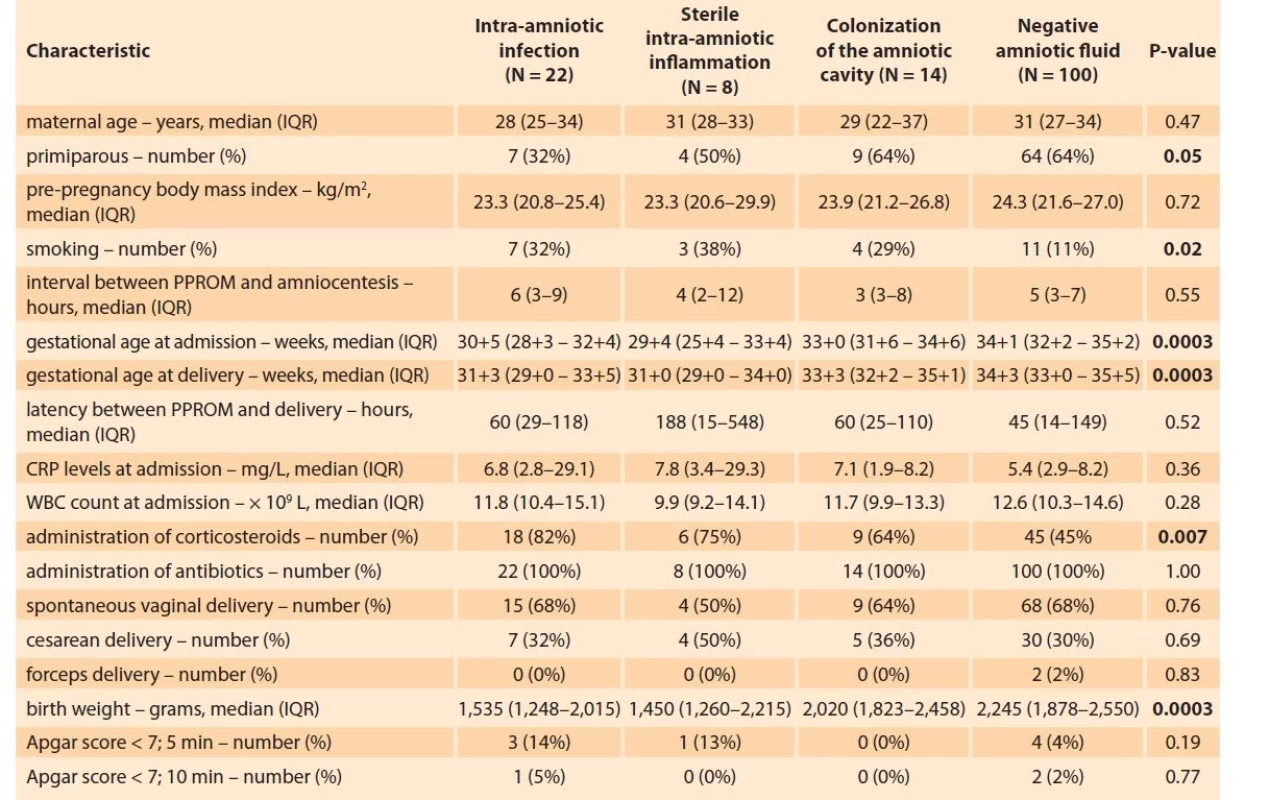
Continuous variables were compared using a nonparametric Kruskal-Wallis test.
Categorical variables were compared using the chi-square test.
Continuous variables are presented as median (IQR) and categorical as number (%).
Statistically significant results are marked in bold.
Amniotic fluid sCD93 according to the presence of MIAC and/or intra-amniotic inflammation
A difference in the levels of sCD93 in amniotic fluid was found among the groups of women with intra-amniotic infection, sterile intra-amniotic inflammation, colonization of the amniotic cavity, and negative amniotic fluid (intra-amniotic infection: median 22.3 ng/mL, IQR 15.0–38.8, sterile intra-amniotic inflammation: median 21.0, IQR 14.2–45.8, colonization of the amniotic cavity: 8.7 ng/mL, IQR 4.5–12.2; negative amniotic fluid: median 8.7 ng/mL, IQR 5.6–13.8) (Fig. 1) in the crude analysis (P < 0.0001) as well as after adjustment for gestational age at sampling, maternal age, and smoking (P = 0.001).
Obr. 1. Hladiny solubilního CD93 v plodové vodě s ohledem na přítomnost intra-amniální infekce, sterilního intra-amniálního zánětu, kolonizace do amniální dutiny a negativní plodovou vodu.

Women with intra-amniotic infection and sterile intra-amniotic inflammation had higher levels of sCD93 in amniotic fluid than those with colonization of the amniotic cavity and with negative amniotic fluid (Tab. 2). No differences in levels of sCD93 in amniotic fluid were identified between women with intra-amniotic infection and sterile intra-amniotic inflammation, as well as between those with colonization of the amniotic cavity and with negative amniotic fluid (Tab. 2).
Tab. 2. Srovnání hladin solubilního CD93 v plodové vodě mezi skupinami žen s intra-amniální infekcí, sterilním intra-amniálním
zánětem, kolonizací do amniální dutiny a negativní plodovou vodou.
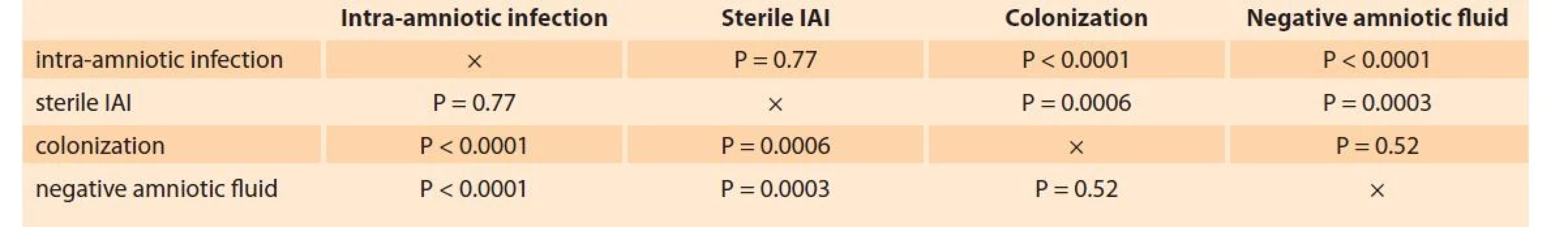
P-value – a comparison between two subgroups (a nonparametric Mann-Whitney U test).
Discussion
Principal findings of the study
1. sCD93 is a constituent of amniotic fluid in pregnancies complicated by PPROM;
2. sCD93 levels in amniotic fluid are elevated in the presence of intra-amniotic inflammation, regardless of the presence or absence of MIAC.
The presence of sCD93 has been proven in various body fluids, including plasma [36,49,50], serum [51], aqueous humor [52], and synovial fluid [57]. In this study, sCD93 was assessed for first time in amniotic fluid. The concentration of sCD93 in amniotic fluid was measurable in each sample, even in those from PPROM pregnancies without the presence of MIAC or intra-amniotic inflammation. Given this finding, sCD93 can be considered as a normal constituent of amniotic fluid in pregnancies complicated by PPROM in the second and third trimesters. In addition, sCD93 seems to be generated and released into amniotic fluid in a constitutive manner. This finding corroborates the observation from an animal study in which the constitutive shedding of CD93 was observed from peritoneal cells [58]. The exact origin of sCD93 in amniotic fluid is not known; however, it is highly likely that amniotic fluid macrophages and neutrophils represent two main sources of sCD93 in amniotic fluid.
Myeloid cells, mainly monocytes and neutrophils, are considered to be one of the main sources of sCD93 [36,37,57–59]. The proteolytic cleavage of the ectodomain of CD93 and subsequent release of sCD93 extracellularly from their surfaces occurs quite rapidly (20 min of stimulation), and it is matrix metalloproteinase-dependent [36]. The generation of sCD93 can be triggered either by an infectious [36,57] or a non-infectious stimulus [37,58,60]. However, sCD93 likely plays a different role in infectious inflammation than in sterile inflammation [57,60]. In infection-driven inflammation, sCD93 serves as a promoter of the inflammatory response [57]. On the other hand, sCD93 attenuates an inflammatory response in sterile inflammation, as shown in sterile inflammation driven by ischemia, which led to tissue damage [60]. In this study, we found higher levels of sCD93 in amniotic fluid from PPROM pregnancies complicated by intra-amniotic inflammation, regardless of the presence of MIAC. The women with intra-amniotic infection and sterile intra-amniotic inflammation had a higher level of sCD93 in amniotic fluid than women with colonization and women without MIAC or intra-amniotic inflammation. The association between the elevation of sCD93 levels and intra-amniotic inflammation can be explained by the increase of neutrophils and macrophages in amniotic fluid in the presence of intra-amniotic inflammation [24,61].
Unfortunately, the data generated by this study cannot address whether amniotic fluid macrophages or neutrophils contribute more to the elevation of sCD93 in amniotic fluid from PPROM pregnancies during the presence of intra-amniotic inflammation. However, a previous study carried out on a mouse model of sterile peritonitis showed that, after stimulation, sCD93 was shed mainly from the surface of macrophages [58]. In addition, an elevation of sCD93 in peritoneal fluid appeared 72 to 96 hours after stimulation despite the fact that the presence of neutrophils in peritoneal fluid was at its highest 24 hours after stimulation and then decreased over time [58]. This finding confirmed that macrophages were the main immune cells in peritoneal fluid during the most intensive production of sCD93 [58].
Strengths and limitations of the study
The strength of this study is the relatively large cohort of women with a well-defined phenotype of spontaneous preterm delivery. Second, the presence of microorganisms in amniotic fluid was assessed by the combination of culture and non-culture techniques. This study also has some limitations. First, the descriptive nature of the study cannot address the origin of sCD93 in amniotic fluid because the membrane counterpart of sCD93 in amniotic fluid cells was not evaluated. In addition, two questions remain unanswered: whether the fetal tissues (placenta and fetal membranes) can express CD93 and whether they may contribute to the generation of CD93 in amniotic fluid.
In conclusion, sCD93 is a constituent of amniotic fluid collected from PPROM pregnancies. The elevation of its level in amniotic fluid in PPROM pregnancies is associated with intra-amniotic inflammation, regardless of the presence of microorganisms and/or their nucleic acids in amniotic fluid.
ORCID authors
M. Kacerovsky 0000-0001-9858-7900
C. Andrys 0000-0001-6489-8786
O. Soucek 0000-0003-3137-4200
R. Kukla 0000-0001-7126-9654
I. Musilova 0000-0002-6960-8319
Submitted/Doručeno: 12. 7. 2022
Accepted/Přijato: 24. 7. 2022
Prof. Marian Kacerovsky, MD, PhD
Department of Obstetrics and Gynecology
Faculty of Medicine,
Charles University
University Hospital Hradec Kralove
Sokolska 581
500 05 Hradec Králové
Zdroje
1. Mercer BM. Preterm premature rupture of the membranes. Obstet Gynecol 2003; 101 (1): 178–193. doi: 10.1016/s0029-7844 (02) 02 366-9.
2. Mercer BM. Preterm premature rupture of the membranes: current approaches to evaluation and management. Obstet Gynecol Clin North Am 2005; 32 (3): 411–428. doi: 10.1016/j.ogc.2005.03.003.
3. Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol 2011; 54 (2): 307–312. doi: 10.1097/GRF. 0b013e318217d4d3.
4. Cobo T, Kacerovsky M, Holst RM et al. Intra-amniotic inflammation predicts microbial invasion of the amniotic cavity but not spontaneous preterm delivery in preterm prelabor membrane rupture. Acta Obstet Gynecol Scand 2012; 91 (8): 930–935. doi: 10.1111/j.1600- 0412.2012.01427.x.
5. Jacobsson B, Mattsby-Baltzer I, Andersch B et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women with preterm prelabor rupture of membranes. Acta Obstet Gynecol Scand 2003; 82 (5): 423–431. doi: 10.1034/j.1600-0412.2003.00157.x.
6. Oh KJ, Lee KA, Sohn YK et al. Intraamniotic infection with genital mycoplasmas exhibits a more intense inflammatory response than intraamniotic infection with other microorganisms in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 2010; 203 (3): 211.e1–211.8. doi: 10.1016/j.ajog.2010.03.035.
7. Cobo T, Kacerovsky M, Palacio M et al. Intra-amniotic inflammatory response in subgroups of women with preterm prelabor rupture of the membranes. PLoS One 2012; 7 (8): e43677. doi: 10.1371/journal.pone.0043677.
8. Musilova I, Kutova R, Pliskova L et al. Intra amniotic inflammation in women with preterm prelabor rupture of membranes. PLoS One 2015; 10 (7): e0133929. doi: 10.1371/journal.pone.0133929.
9. Romero R, Miranda J, Chaemsaithong P et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2015; 28 (12): 1394–1409. doi: 10.3109/14767058.2014. 958463.
10. DiGiulio DB, Romero R, Kusanovic JP et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol 2010; 64 (1): 38–57. doi: 10.1111/j.1600-0897.2010.00830.x.
11. Dulay AT, Buhimschi CS, Zhao G et al. Soluble TLR2 is present in human amniotic fluid and modulates the intraamniotic inflammatory response to infection. J Immunol 2009; 182 (11): 7244–7253. doi: 10.4049/jimmunol.0803517.
12. Kacerovsky M, Andrys C, Hornychova H et al. Amniotic fluid soluble Toll-like receptor 4 in pregnancies complicated by preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med 2012; 25 (7): 1148–1155. doi: 10.3109/ 14767058.2011.626821.
13. Mantovani A, Garlanda C, Bottazzi B. Pentraxin 3, a non-redundant soluble pattern recognition receptor involved in innate immunity. Vaccine 2003; 21 (Suppl 2): S43–S47. doi: 10.1016/s0264-410x (03) 00199-3.
14. Stahl PD, Ezekowitz RA. The mannose receptor is a pattern recognition receptor involved in host defense. Curr Opin Immunol 1998; 10 (1): 50–55. doi: 10.1016/s0952-7915 (98) 80031-9.
15. Imler JL, Hoffmann JA. Signaling mechanisms in the antimicrobial host defense of Drosophila. Curr Opin Microbiol 2000; 3 (1): 16–22. doi: 10.1016/s1369-5274 (99) 00045-4.
16. Cruciani L, Romero R, Vaisbuch E et al. Pentraxin 3 in amniotic fluid: a novel association with intra-amniotic infection and inflammation. J Perinat Med 2010; 38 (2): 161–171. doi: 10.1515/jpm.2009.141.
17. Mazaki-Tovi S, Romero R, Vaisbuch E et al. Adiponectin in amniotic fluid in normal pregnancy, spontaneous labor at term, and preterm labor: a novel association with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2010; 23 (2): 120–130. doi: 10.3109/14767050903026481.
18. Cobo T, Palacio M, Martínez-Terrón M et al. Clinical and inflammatory markers in amniotic fluid as predictors of adverse outcomes in preterm premature rupture of membranes. Am J Obstet Gynecol 2011; 205 (2): 126.e1–126.e8. doi: 10.1016/j.ajog.2011.03.050.
19. Rosenberg VA, Buhimschi IA, Dulay AT et al. Modulation of amniotic fluid activin-a and inhibin-a in women with preterm premature rupture of the membranes and infection-induced preterm birth. Am J Reprod Immunol 2012; 67 (2): 122–131. doi: 10.1111/j.1600-0897. 2011.01074.x.
20. Kacerovsky M, Andrys C, Drahosova M et al. Soluble Toll-like receptor 1 family members in the amniotic fluid of women with preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med 2012; 25 (9): 1699–1704. doi: 10.3109/14767058.2012.658463.
21. Tambor V, Kacerovsky M, Andrys C et al. Amniotic fluid cathelicidin in PPROM pregnancies: from proteomic discovery to assessing its potential in inflammatory complications diagnosis. PLoS One 2012; 7 (7): e41164. doi: 10.1371/journal.pone.0041164.
22. Andrys C, Kacerovsky M, Drahosova M et al. Amniotic fluid soluble Toll-like receptor 2 in pregnancies complicated by preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2013; 26 (5): 520–527. doi: 10.3109/14767 058.2012.741634.
23. Romero R, Kadar N, Miranda J et al. The diagnostic performance of the Mass Restricted (MR) score in the identification of microbial invasion of the amniotic cavity or intra-amniotic inflammation is not superior to amniotic fluid interleukin-6. J Matern Fetal Neonatal Med 2014; 27 (8): 757–769. doi: 10.3109/14767058.2013.844 123.
24. Gomez-Lopez N, Romero R, Garcia-Flores V et al. Amniotic fluid neutrophils can phagocytize bacteria: a mechanism for microbial killing in the amniotic cavity. Am J Reprod Immunol 2017; 78 (4). doi: 10.1111/aji.12723.
25. Gomez-Lopez N, Romero R, Xu Y et al. Neutrophil extracellular traps in the amniotic cavity of women with intra-amniotic Infection: a new mechanism of host defense. Reprod Sci 2017; 24 (8): 1139–1153. doi: 10.1177/19337191166 78690.
26. Gomez-Lopez N, Romero R, Xu Y et al. The immunophenotype of amniotic fluid leukocytes in normal and complicated pregnancies. Am J Reprod Immunol 2018; 79 (4): e12827. doi: 10.1111/aji.12827.
27. Bernard A, Boumsell L. Human leukocyte differentiation antigens. Presse Med 1984; 13 (38): 2311–2316.
28. Fiebig H, Behn I, Gruhn R et al. Characterization of a series of monoclonal antibodies against human T cells. Allerg Immunol (Leipz) 1984; 30 (4): 242–250.
29. Zola H, Swart B, Nicholson I et al. CD molecules 2005: human cell differentiation molecules. Blood 2005; 106 (9): 3123–3126. doi: 10.1182/ blood-2005-03-1338.
30. Zola H, Swart B, Banham A et al. CD molecules 2006 – human cell differentiation molecules. J Immunol Methods 2007; 319 (1–2): 1–5. doi: 10.1016/j.jim.2006.11.001.
31. Chan JK, Ng CS, Hui PK. A simple guide to the terminology and application of leucocyte monoclonal antibodies. Histopathology 1988; 12 (5): 461–480. doi: 10.1111/j.1365-2559.1988.tb01967.x.
32. Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 1989; 74 (7): 2527–2534.
33. Arribas J, Coodly L, Vollmer P et al. Diverse cell surface protein ectodomains are shed by a system sensitive to metalloprotease inhibitors. J Biol Chem 1996; 271 (19): 11376–11382. doi: 10.1074/jbc.271.19.11376.
34. Galkina E, Tanousis K, Preece G et al. L-selectin shedding does not regulate constitutive T cell trafficking but controls the migration pathways of antigen-activated T lymphocytes. J Exp Med 2003; 198 (9): 1323–1335. doi: 10.1084/jem. 20030485.
35. Blobel CP. Remarkable roles of proteolysis on and beyond the cell surface. Curr Opin Cell Biol 2000; 12 (5): 606–612. doi: 10.1016/s0955-067 4 (00) 00139-3.
36. Bohlson SS, Silva R, Fonseca MI et al. CD93 is rapidly shed from the surface of human myeloid cells and the soluble form is detected in human plasma. J Immunol 2005; 175 (2): 1239–1247. doi: 10.4049/jimmunol.175.2.1239.
37. Blackburn JW, Lau DH, Liu EY et al. Soluble CD93 is an apoptotic cell opsonin recognized by alpha x beta 2. Eur J Immunol 2019; 49 (4): 600–610. doi: 10.1002/eji.201847801.
38. Greenlee-Wacker MC, Briseno C, Galvan M et al. Membrane-associated CD93 regulates leukocyte migration and C1q-hemolytic activity during murine peritonitis. J Immunol 2011; 187 (6): 3353–3361. doi: 10.4049/jimmunol.1100803.
39. Khan KA, Naylor AJ, Khan A et al. Multimerin-2 is a ligand for group 14 family C-type lectins CLEC14A, CD93 and CD248 spanning the endothelial pericyte interface. Oncogene 2017; 36 (44): 6097–6108. doi: 10.1038/onc.2017. 214.
40. Fakhari S, Bashiri H, Ghaderi B et al. CD93 is selectively expressed on human myeloma cells but not on B lymphocytes. Iran J Immunol 2019; 16 (2): 142–150. doi: 10.22034/IJI.2019.80257.
41. McGreal EP, Ikewaki N, Akatsu H et al. Human C1qRp is identical with CD93 and the mNI-11 antigen but does not bind C1q. J Immunol 2002; 168 (10): 5222–5232. doi: 10.4049/jimmunol.168.10.5222.
42. Ghebrehiwet B, Peerschke EI. cC1q-R (calreticulin) and gC1q-R/p33: ubiquitously expressed multi-ligand binding cellular proteins involved in inflammation and infection. Mol Immunol 2004; 41 (2–3): 173–183. doi: 10.1016/j.molimm.2004.03.014.
43. McGreal E, Gasque P. Structure-function studies of the receptors for complement C1q. Biochem Soc Trans 2002; 30 (Pt 6): 1010–1014. doi: 10.1042/bst0301010.
44. Bohlson SS, Zhang M, Ortiz CE et al. CD93 interacts with the PDZ domain-containing adaptor protein GIPC: implications in the modulation of phagocytosis. J Leukoc Biol 2005; 77 (1): 80–89. doi: 10.1189/jlb.0504305.
45. Zhang M, Bohlson SS, Dy M et al. Modulated interaction of the ERM protein, moesin, with CD93. Immunology 2005; 115 (1): 63–73. doi: 10.1111/j.1365-2567.2005.02120.x.
46. Ikewaki N, Kulski JK, Inoko H. Regulation of CD93 cell surface expression by protein kinase C isoenzymes. Microbiol Immunol 2006; 50 (2): 93–103. do: 10.1111/j.1348-0421.2006.tb03774.x.
47. Greenlee MC, Sullivan SA, Bohlson SS. CD93 and related family members: their role in innate immunity. Curr Drug Targets 2008; 9 (2): 130–138. doi: 10.2174/138945008783502421.
48. Galvagni F, Nardi F, Spiga O et al. Dissecting the CD93-Multimerin 2 interaction involved in cell adhesion and migration of the activated endothelium. Matrix Biol 2017; 64: 112–127. doi: 10.1016/j.matbio.2017.08.003.
49. Mälarstig A, Silveira A, Wågsäter D et al. Plasma CD93 concentration is a potential novel biomarker for coronary artery disease. J Intern Med 2011; 270 (3): 229–236. doi: 10.1111/j.1365- 2796.2011.02364.x.
50. Youn JC, Yu HT, Jeon JW et al. Soluble CD93 levels in patients with acute myocardial infarction and its implication on clinical outcome. PLoS One 2014; 9 (5): e96538. doi: 10.1371/journal.pone.0096538.
51. Park HJ, Han H, Lee SC et al. Soluble CD93 in serum as a marker of allergic inflammation. Yonsei Med J 2017; 58 (3): 598–603. doi: 10.3349/ymj.2017.58.3.598.
52. Tosi GM, Caldi E, Parolini B et al. CD93 as a potential target in neovascular age-related macular degeneration. J Cell Physiol 2017; 232 (7): 1767–1773. doi: 10.1002/jcp.25689.
53. Strawbridge RJ, Hilding A, Silveira A et al. Soluble CD93 is involved in metabolic dysregulation but does not influence carotid intima-media thickness. Diabetes 2016; 65 (10): 2888–2899. doi: 10.2337/db15-1333.
54. Musilova I, Andrys C, Holeckova M et al. Interleukin-6 measured using the automated electrochemiluminescence immunoassay method for the identification of intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2018; 33 (11): 1919–1926. doi: 10.1080/14767058.2018. 1533947.
55. Fouhy F, Deane J, Rea MC et al. The effects of freezing on faecal microbiota as determined using MiSeq sequencing and culture-based investigations. PloS One 2015; 10 (3): e0119355. doi: 10.1371/journal.pone.0119355.
56. Greisen K, Loeffelholz M, Purohit A et al. PCR primers and probes for the 16S rRNA gene of most species of pathogenic bacteria, including bacteria found in cerebrospinal fluid. J Clin Microbiol 1994; 32 (2): 335–351. doi: 10.1128/jcm.32.2.335-351.1994.
57. Jeon JW, Jung JG, Shin EC et al. Soluble CD93 induces differentiation of monocytes and enhances TLR responses. J Immunol 2010; 185 (8): 4921–4927. doi: 10.4049/jimmunol.0904011.
58. Greenlee MC, Sullivan SA, Bohlson SS. Detection and characterization of soluble CD93 released during inflammation. Inflamm Res 2009; 58 (12): 909–919. doi: 10.1007/s00011- 009-0064-0.
59. Ikewaki N, Tamauchi H, Inoko H. Decrease in CD93 (C1qRp) expression in a human monocyte-like cell line (U937) treated with various apoptosis-inducing chemical substances. Microbiol Immunol 2007; 51 (12): 1189–1200. doi: 10.1111/j.1348-0421.2007.tb04014.x.
60. Harhausen D, Prinz V, Ziegler G et al. CD93/AA4.1: a novel regulator of inflammation in murine focal cerebral ischemia. J Immunol 2010; 184 (11): 6407–6417. doi: 10.4049/ jimmunol.0902342.
61. Gomez-Lopez N, Romero R, Xu Y et al. Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? Am J Obstet Gynecol 2017; 217 (6): 693.e1–693.e16. doi: 10.1016/ j.ajog.2017.09.013.
Štítky
Dětská gynekologie Gynekologie a porodnictví Reprodukční medicínaČlánek vyšel v časopise
Česká gynekologie

2022 Číslo 6
Nejčtenější v tomto čísle
- A novel estetrol-containing combined oral contraceptive: European expert panel review
- Endometriosis in postmenopause
- Selected pathological conditions affecting endometrial receptivity
- Vulvar carcinoma and its recurrences – principles of surgical treatment
