Matrix metalloproteinases MMP-2 and MMP-9 as markers for the prediction of preeclampsia in the first trimester
Matrixové metaloproteinázy MMP-2 a MMP-9 jako markery predikce preeklampsie v prvním trimestru
Úvod: Preeklampsie je onemocnění, které ohrožuje na životě matku I plod. Na celém světě je každý rok diagnostikováno u 10 milionů žen, což odpovídá 3–8 % všech těhotenství. V současné době nemá preeklampsie prokázanou účinnou léčbu. To zdůrazňuje potřebu předpovědi této komplikace. Tato prospektivní studie se provádí za účelem stanovení, zda mají matrixové metaloproteinázy-2 a -9 prognostický význam jako časné markery preeklampsie. Materiály a metody: Hladiny matrixových metaloproteináz-2 a -9 byly hodnoceny u 72 pacientek. U 34 z nich se následně v průběhu těhotenství vyvinula preeklampsie (u 20 pacientek mírná forma, u 14 pacientek těžká forma). Tyto pacientky představovaly základní skupinu. Kontrolní skupinu tvořilo 38 pacientek. Výsledky: U těhotných žen, u nichž se následně vyvinula preeklampsie, byla hladina matrixové metaloproteinázy-2 v 11.–13. týdnu těhotenství 155 ± 73,4 ng/ml a byla významně vyšší než úroveň u těhotných žen bez hypertenzních poruch (75,0 ± 32,8 ng/ml). Prováděná studie prokazuje významně nižší koncentraci matrixové metaloproteinázy 9 u těhotných žen s preeklampsií v porovnání s kontrolní skupinou (749 ± 296 ng/ml vs. 1 667 ± 552 ng/ml; p < 0,001). Provedený výzkum ukazuje, že v prvním trimestru byla mezní hodnota matrixové metaloproteinázy-2 pro predikci rozvoje preeklampsie ≥ 102 ng/ml (senzitivita 88,24 % a specificita 82,76 %). V případě matrixové metaloproteinázy-9 předpovídala v prvním trimestru rozvoj preeklampsie hladina ≤ 980 ng/ml se senzitivitou 85,29 % a specificitou 84,48 %. Závěr: Studie stanovila mezní hodnoty matrixových metaloproteináz-2 a -9 v prvním trimestru pro předpověď rozvoje preeklampsie.
Klíčová slova:
těhotenství – preeklampsie – matrixové metaloproteinázy – predikce preeklampsie
Authors:
Timokhina E. 1; Zinin V. 2; Ignatko I. 1; Ibragimova S. 1; Belotserkovtseva L. 3; Strizhakov A. 1
Authors‘ workplace:
Department of Obstetrics, Gynecology and Perinatology, I. M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russian Federation
1; Department of Gravitational Blood Surgery, Surgut Clinical Perinatal Center, Surgut, Russian Federation
2; Department of Obstetrics, Gynecology and Perinatology, Surgut State University, Surgut, Russian Federation
3
Published in:
Ceska Gynekol 2021; 86(4): 228-235
Category:
doi:
https://doi.org/10.48095/cccg2021228
Overview
Introduction: Preeclampsia is a life-threatening condition for the mother and foetus. Globally, it is diagnosed in 10 mil. women every year, which accounts for 3% to 8% of all pregnancies. Currently there is no proven effective treatment for preeclampsia. The aforesaid text actualises the issue of predicting this complication. To determine the prognostic significance of matrix metalloproteinases-2 and -9 levels as early markers of preeclampsia, the present prospective study was conducted. Materials and methods: The levels of matrix metalloproteinases-2 and -9 were assessed in 72 patients. Thirty-four of them subsequently developed preeclampsia during pregnancy (20 patients with moderate preeclampsia, 14 patients with severe preeclampsia), and constituted the basic group; 38 patients made up the control group. Results: In pregnant women with the subsequent development of preeclampsia, the level of matrix metalloproteinase-2 at 11–13 weeks of gestation was 155 ± 73.4 ng/mL and significantly exceeded its level in pregnant women without hypertensive disorders – 75.0 ± 32.8 ng/mL. The study conducted demonstrates a significantly lower concentration of matrix metalloproteinase-9 in pregnant women with preeclampsia compared to the control – 749 ± 296 ng/mL and 1,667 ± 552 ng/mL (P < 0.001). The performed research figures that in the first trimester, the cut-off value of matrix metalloproteinase-2 for predicting the development of preeclampsia is ≥ 102 ng/mL (sensitivity 88.24% and specificity 82.76%). For matrix metalloproteinase-9, a level of ≤ 980 ng/mL in the first trimester predicts the development of preeclampsia with a sensitivity of 85.29% and a specificity of 84.48%. Conclusion: The study established the cut-off values of matrix metalloproteinases-2 and -9 for predicting the development of preeclampsia in the first trimester.
Keywords:
pregnancy – preeclampsia – matrix metalloproteinases – prediction of preeclampsia
Introduction
Preeclampsia (PE) is a life-threatening condition for the mother and foetus. Globally, it is diagnosed in 10 million women every year, which accounts for 3–8% of all pregnancies. It is the cause of death of 76,000 pregnant women and 500,000 foetuses and newborns annually worldwide [1–3]. In the structure of maternal mortality in developed countries, PE steadily occupies a leading position, accounting for up to 20% of all causes of maternal mortality [4]. Severe PE complicates 1.4% of all pregnancies worldwide. This condition usually develops long before term pregnancy and therefore requires early delivery. This leads to the birth of a premature newborn, often with growth retardation and with all the consequences of prematurity [1–4].
The financial impact of PE must be mentioned. For example, in the United States, the total cost of PE treatment, which includes intensive care units for mothers and newborns and subsequent rehabilitation reaches $2.18 bil., which is one-third of all costs for postnatal care [5].
The next aspect of the impact of PE on population health is the long-term risks for mother and child. PE experienced during pregnancy significantly increases the risk of long-term cardiovascular diseases (myocardial infarction, stroke), metabolic disorders (both type I and II diabetes mellitus) and sudden death in the future [2,6]. There is evidence that hypertensive disorders during pregnancy, especially PE, considerably expand the risk of mental disorders later in children [7].
There is no confirmed effective treatment for PE to date. The only exception is delivery, which in the case of preterm pregnancy means premature birth and all the problems of a premature newborn, especially with extremely low body weight. In this regard, the issue of predicting PE is relevant.
It was found that the main pathological processes leading to the development of PE occur as early as the first trimester. This is a sequential violation of the syncytiotrophoblast invasion and defective remodelling of the spiral arteries, followed by placenta oxidative stress and an imbalance of proangiogenic and antiangiogenic factors [1,3,8]. Extracellular proteolytic enzymes – matrix metalloproteinases (MMP) of the 2, 3, 7, 9, 13 types [9,10], capable of destroying the components of the extracellular matrix [10,11] are involved in these crucial stages of pregnancy development. For successful implantation, MMPs produced by trophoblast cells play an important role: MMP-2, -3, -7, -9, -13 [5,6], MMP-2 and MMP-9 are the key effectors of this process [9–11]. MMP-2, MMP-9, MMP-3 and MMP-13 are involved in the remodelling of the spiral arteries. They ensure the degradation of endothelin and adrenomedullin, which leads to vasodilation of the spiral arteries and the establishment of adequate placental perfusion in the future.
This way, several studies indicate the involvement of MMPs in the key processes of the first trimester of pregnancy, which determine the subsequent development of PE [9–12]. At the same time, there are no studies on the practical application of MMP in predicting the development of PE as yet. There are few works [13–15] that confirm the direct correlation of changes in the level of MMP-2 and the inverse correlation of MMP-9 with the development of PE. However, they do not suggest any real guidelines for use in clinical practice.
In this regard, research was carried out to prove the predictive power of MMP-2 and -9 in the development of PE and their threshold concentrations were established with high sensitivity and specificity, which can be advisory for practical use.
Materials and Methods
Study design
To determine the predictive significance of MMP-2 and MMP-9 as early markers of PE, a prospective study was conducted involving 310 pregnant women aged 18–45 years with singleton pregnancies. Patients were enrolled at 10–11 weeks of gestational age.
The criteria for inclusion were the informed consent of the patient to participate in the study, age over 18 years, singleton pregnancy.
Criteria for exclusion from the study were: pregnancy after assisted reproductive technologies, multiple pregnancy, connective tissue dysplasia, type I or II diabetes mellitus, oncological diseases, autoimmune diseases, infectious diseases (HIV infection, hepatitis), administration of medications, or psychoactivators, smoking or drug addiction.
All patients underwent venous blood sampling to determine the concentration of MMP-2 and MMP-9 types at 11–13 weeks of gestational age. The sampling was carried out simultaneously with the first screening test. The patients remained under supervision throughout the pregnancy until delivery. The collected blood samples were taken into a procession after the end of the pregnancy period. Patients with PE-complicated pregnancy – 34 women – constituted the basic group.
Informed consent was obtained from each woman to participate in the study and to collect venous blood samples. The study protocol was approved by the Sechenov University Ethics Committee (No 10–17 of 16 November 2017), FSAEI HE I. M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia.
During the study, 18 patients (5.8%) were withdrawn. Six of these had a high risk of foetal chromosomal abnormalities following the results of the ultrasound investigation, biochemical screening and noninvasive prenatal test (N = 6). One patient had a congenital foetal defect (N = 1). In this regard, the indicated patients underwent therapeutic abortion. Eleven patients had a spontaneous miscarriage before 22 weeks of gestation. In 29 patients (8.7%) pregnancy ended with preterm delivery, not associated with PE and/or placental insufficiency (FGR – fetal growth restriction).
Of all the pregnant women under supervision, PE was detected in 34 patients (10.9%). Of these, in 14 patients (41.2%) PE was regarded as PE with severe features.
Preeclampsia refers to the new onset of hypertension and proteinuria or the new onset of hypertension and significant end-organ dysfunction with or without proteinuria after 20 weeks of gestation or postpartum in a previously normotensive woman [2].
The diagnosis of preeclampsia with severe features (formerly severe preeclampsia) is made in the subset of women with preeclampsia who have severe hypertension and/or specific signs or symptoms of significant end-organ dysfunction that signify the severe end of the preeclampsia spectrum:
- Systolic blood pressure of 160 mmHg or higher or a diastolic blood pressure of 110 mmHg or higher on two occasions at least 4 hours apart while the patient was on bed rest (unless antihypertensive therapy was initiated prior to this time);
- Thrombocytopenia (platelet count less than 100,000/μL);
- Impaired liver function as indicated by abnormally elevated blood concentrations of liver enzymes (to twice the normal concentration), severe persistent right-upper quadrant or epigastric pain unresponsive to medication and not accounted for by alternative diagnoses, or both;
- Progressive renal insufficiency (a serum creatinine concentration greater than 1.1 mg/dL or a doubling of the serum creatinine concentration in the absence of other renal disease);
- Pulmonary oedema;
- New-onset cerebral or visual disturbances.
Pregnant women with onset PE made up the basic group – 34 patients. In 229 remaining patients, pregnancy proceeded without hypertensive disorders and ended in term delivery. Among these patients, 38 were selected by simple randomisation for subsequent determination of the concentration of MMP-2 and MMP-9 in the blood. This group constituted the control – 38 observations. Thus, the total number of patients with analysed levels of MMP-2 and MMP-9 comprised 72 pregnant women.
Laboratory studies
The blood samples taken at 11–13 weeks of gestation were placed in sterile tubes at a temperature of 40 °C and centrifuged for 15 minutes at 1,500 rpm. Afterwards, plasma samples were stored at –800 °С until analysis. The analysis to determine the level of MMP-2 and MMP-9 was performed after delivery when the patients were divided into groups based on the development of PE or uncomplicated pregnancy.
MMP-2 and -9 levels were determined using the enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s protocol (Cloud-CloneCorp. Houston, USA).
The kits used were designed for the quantitative determination of MMP-2 (SEA100Hu, minimum detectable concentration 0.27 ng/mL) and MMP-9 (SEA553Hu, minimum detectable concentration 0.055 ng/mL) by ELISA. The collection, storage and analysis of samples were carried out following the requirements of the manufacturer of the reagents, Cloud-Clone Corp. Houston, USA.
Statistical analysis
In comparing risk factors and individual characteristics of the studied groups, a parametric method of comparing the two groups with the calculation of the Student’s t-test was used.
The obtained values of the Student’s t-test were evaluated by comparison with the critical values. Differences in indicators were considered statistically significant at a confidence level of P < 0.05.
The comparison of percentages was carried out using the Pearson c2 test.
The significance of differences in MMP concentrations in the studied groups (due to the lack of normal distribution attributes) was assessed using the Mann-Whitney U-test. Differences were considered statistically significant at P < 0.05.
The ROC (receiver operating characteristic) analysis was suggested for assessment of the prognostic significance of the MMP concentration in the first trimester for predicting PE development. It ensured the definition of the sensitivity, specificity and predictive accuracy of positive and negative results for MMP-2 and MMP-9.
ROC analysis also allowed in determining the cut-off values of MMP-2 and MMP-9 to predict the development of PE.
Statistical analysis was performed using SPSS Statistics 20 and Jamovi 1.1.9 software.
Results
In general, 72 patients were subject to MMP-2 and MMP-9 level assessment. 34 of them subsequently developed PE during pregnancy (20 patients – mild PE, 14 patients – severe PE) constituting the basic group. 38 patients, whose pregnancy proceeded without hypertensive disorders and ended in term delivery, made up the control group.
Clinical characteristics
The clinical characteristics of the studied patients are presented in Tab. 1.
Tab. 1. Klinické charakteristiky hodnocených skupin.
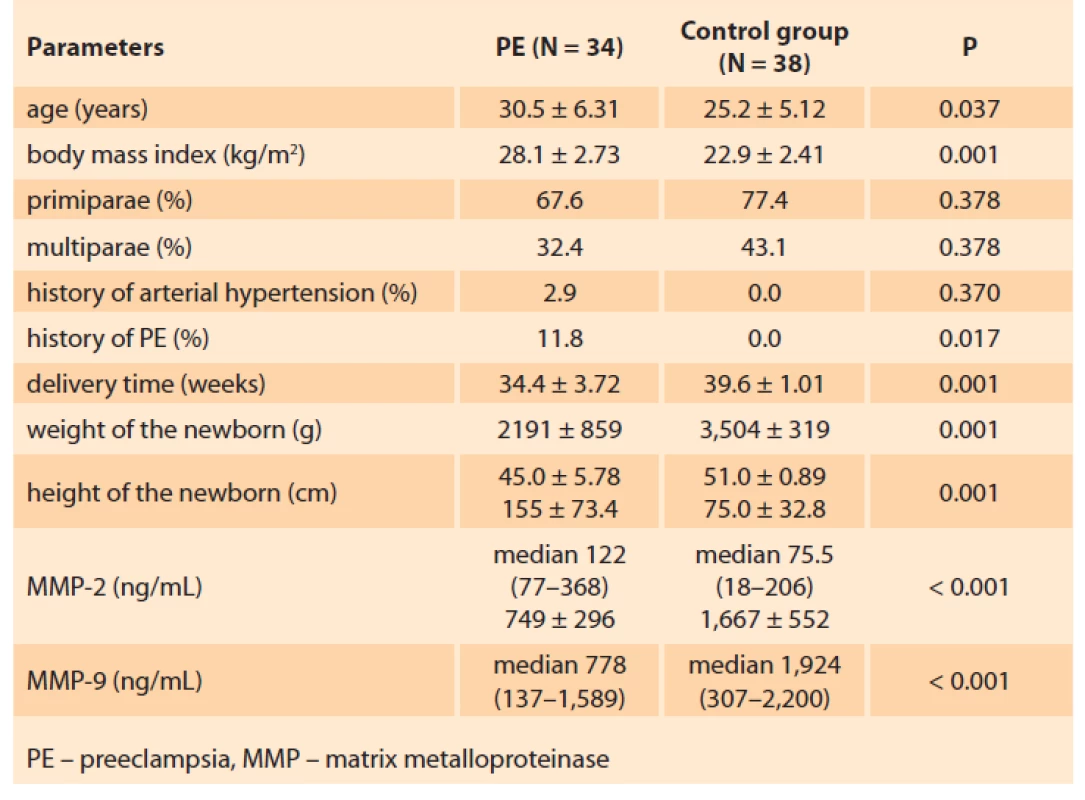
The average age of the women in the basic group was 30.5 ± 6.31 years, being significantly higher than that of the patients in the control group 25.2 ± 5.12 years.
The body mass index in the basic group was significantly higher (28.1 ± 2.73 kg/m2) than in the control group: 22.9 ± 2.41 kg/m2 (P ≤ 0.001).
The incidence of previous chronic arterial hypertension (AH) in the patients of the basic group totalled 2.9%.
In the basic group, primiparae accounted for 67.6% and 77.4% in the control group; the differences were statistically insignificant (P < 0.05). Multiparae comprised 32.4% and 22.6%, respectively.
It should be noted that among the multiparae of the basic group, 11.8% experienced PE complication during the primipregnancy as well.
The delivery time for the patients of the basic group was 34.4 ± 3.72 weeks, 39.6 ± 1.01 weeks for the control group (P < 0.001).
The features of the PE in patients of the basic group are presented in Tab. 2.
Tab. 2. Znaky PE u pacientek v základní skupině (n = 34).
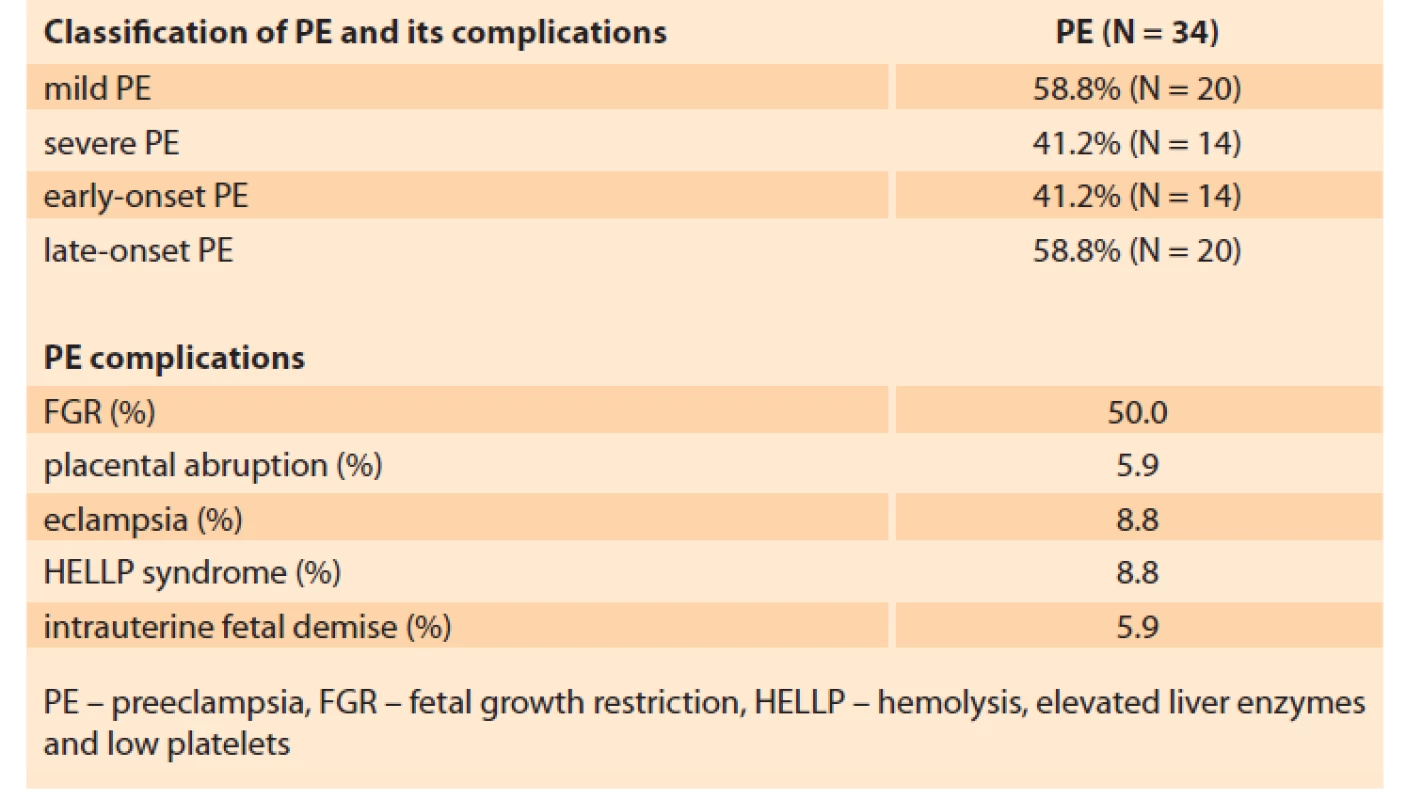
In 41.2% of the patients PE was regarded as early-onset, i.e. it developed and required delivery before 34 weeks of gestation (30.7 ± 2.63 weeks). For 58.8% of the patients, delivery was required after 34 weeks (37.8 ± 1.53 weeks), which counts for late-onset PE.
Of all the PE patients, 41.2% had symptoms of severe PE.
The present study distinguishes the following PE complications: placental abruption 5.9%, development of eclampsia 8.8%, HELLP-syndrome 8.8%, antenatal foetal death 5.9%.
In one-half of the patients, PE was combined with placental insufficiency and foetal growth retardation.
MMP plasma level study results
MMP-2 and MMP-9 concentrations at a gestational age of 11–13 weeks in the pregnant women studied are presented in Tab. 1.
ММР-2
In pregnant women who subsequently developed PE, the MMP-2 level at 11–13 weeks of gestation was 155 ± 73.4 ng/mL. This significantly exceeds the level of MMP-2 in pregnant women, in whom further pregnancy proceeded without hypertensive disorders: 75.0 ± 32.8 ng/mL (P < 0.001) (Fig. 1).
Obr. 1. Plazmatická koncentrace MMP-2
v prvním trimestru u pacientek, u nichž
se následně vyvinula PE, nebo měly fyziologické
těhotenství. Krabicový graf
znázorňuje medián (tučná horizontální
čára), interkvartilové rozpětí (krabice)
a celkové rozmezí (vousy). Hvězdička
znázorňuje p < 0,001.

MMP – matrixová metaloproteináza
ММР-9
The study established a significantly lower MMP-9 concentration in the basic group of patients compared to the control group, namely 749 ± 296 ng/mL and 1,667 ± 552 ng/mL (P < 0.001) (Fig. 2).
Obr. 2. Plazmatická koncentrace MMP-9
v prvním trimestru u pacientek, u nichž
se následně vyvinula PE, nebo měly fyziologické
těhotenství. Krabicový graf
znázorňuje medián (tučná horizontální
čára), interkvartilové rozpětí (krabice)
a celkové rozmezí (vousy). Hvězdička
znázorňuje p < 0,001.

MMP – matrixová metaloproteináza
MMP-9 level was lower in the basic group compared to the control group, the differences being statistically significant (P < 0.001).
ROC analysis applied for the determination of the MMP cut-off for PE predicting
To establish MMP-2 and MMP-9 prognostic value in terms of PE development, ROC analysis was performed. The results are presented in Tab. 3.
Tab. 3. Analýza křivky ROC pro MMP-2 a MMP-9.
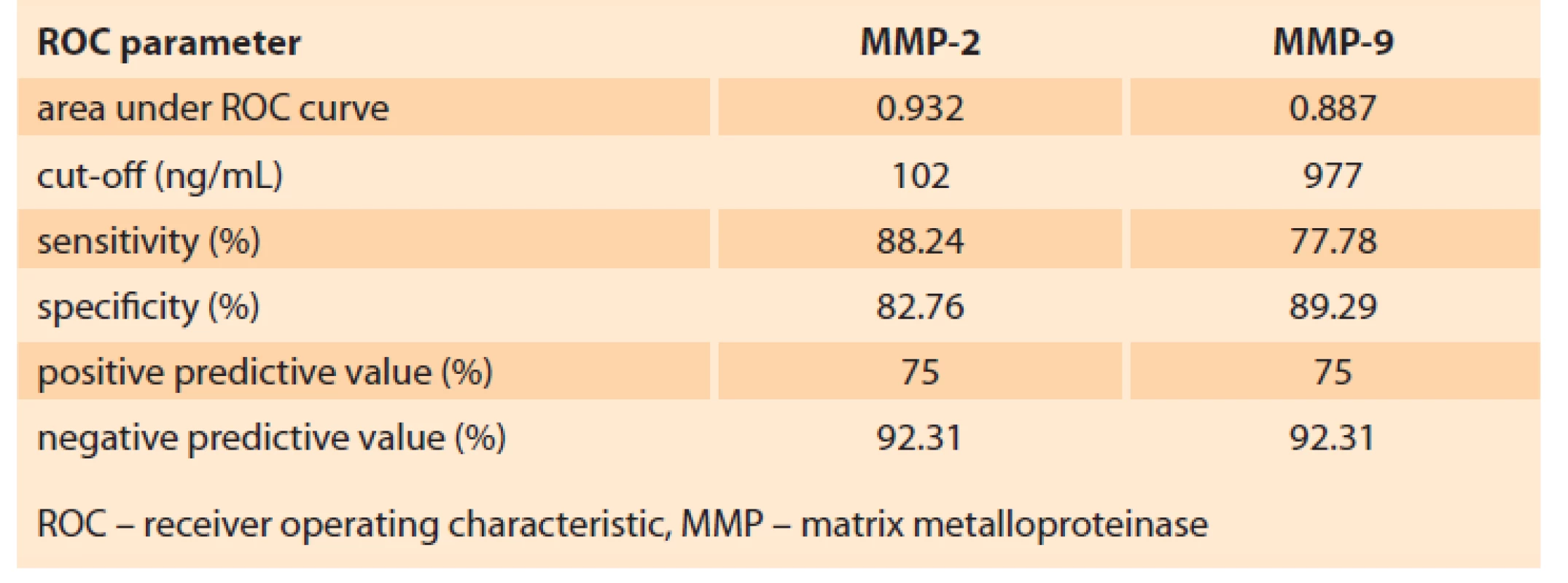
Taking the predictive point of MMP-2 and MMP-9 blood concentration at 11–13 weeks of gestation, the ROC analysis allowed establishing cut-off values (Tab. 3, Fig. 3A, B).
Obr. 3. ROC analýza pro koncentraci MMP v prvním trimestru, ng/ml: A) MMP-2, B) ММР: plocha pod křivkou (AUC) pro křivku
ROC.

The study conducted establishes MMP-2 and MMP-9 threshold concentrations for predicting PE.
According to the results of the ROC analysis for MMP-2, the cut-off value was ≥ 102 ng/mL, which predicts the development of PE with a sensitivity of 88.24% and a specificity of 82.76% (Tab. 3).
The performed ROC analysis allowed determining MMP-9 ≤ 980 ng/mL as the threshold concentration for predicting PE with a sensitivity of 85.29% and a specificity of 84.48% (Tab. 3).
Discussion
PE remains a complex and unsolved problem today.
First, this complication in both developed and emerging countries of the world manifests no tendency to decrease. Even with a high level of healthcare system development, it remains one of the leading causes of maternal mortality [2,4].
Secondly, the pathophysiological mechanisms underlying PE are laid down in the first trimester – many weeks BEFORE the first symptoms of this complication appear. Deviations from the normal course of implantation processes, trophoblast invasion, gestational transformation of the spiral arteries (essential for adequate foetal growth and development) and adaptation of the maternal organism to pregnancy further lead to PE. In the first trimester, violations of these processes do not have any clinical manifestations that would give clinicians cause for concern. However, the pregnant woman is already at risk, which is realised in the second half of the pregnancy period.
Thirdly, with an established diagnosis of PE, there are no effective methods for its treatment. The only approach is delivery since only the removal of foetus and placenta will stop the cascade of numerous launched mechanisms that damage the vessels and target organs.
Given the above, a search for PE predictors with high prediction specificity is crucial, directly from the first weeks of pregnancy. The prediction of PE is based on the identification of risk factors, assessment of ultrasound parameters and biochemical markers [1–3,8].
Currently, existing biochemical markers (PlGF – placental growth factor, endothelin, placental protein-13 [PP-13]) are characterised by insufficient sensitivity and specificity [1,3,8]).
In this regard, it is urgently necessary to search for early PE markers with high sensitivity and specificity effective from the first trimester so far.
Since MMPs are involved in the key processes of the first trimester of pregnancy, ensuring its normal subsequent development (implantation, invasion of trophoblast, gestational transformation of the spiral arteries), an increase or decrease in the MMP level indicates a violation of the course of these processes. Therefore, the change in the concentration and assessment of the activity of metalloproteinases makes MMPs a subject of study as potential early markers of PE.
The present study showed that at 11–13 weeks of gestation in the group of pregnant women who subsequently develop PE, the concentration of MMP-2 is significantly higher compared to physiological pregnancy (155 ± 73.4 ng/mL and 75.0 ± 32.8 ng/mL (P < 0.001).
Conversely, the concentration of MMP-9 in the group of pregnant women with subsequent PE was significantly lower than in the control group: 749 ± 296 ng/mL and 1,667 ± 552 ng/mL (P < 0.001), respectively.
MMP-2 and MMP-9 are known to be involved in the processes of blastocyst implantation. They are responsible for the destruction of the collagen matrix and the successful invasion of the extravillous cytotrophoblast into the endometrium. Their deficiency can disrupt this key process of pregnancy and further lead to disruption of the chorion and placenta formation [9,11].
This is confirmed by the results of the present work.
MMP-9 participates in gestational remodelling of the spiral arteries. The revealed decreased level of MMP-9 in pregnant women with the subsequent development of PE confirms that a decrease in this enzyme disrupts the physiological transformation of the spiral arteries, which further leads to PE development. Therefore, a decrease in the MMP-9 level at 11–13 weeks of gestation can be considered a marker for predicting PE.
The leading role in implantation belongs to MMP-2. Chen et al in their research studied the role of MMP-2 and MMP-9 in the placenta of rats in the early days of pregnancy. The study detected a correlation between a decrease in MMP-2 and MMP-9 and a defect in trophoblast invasion, insufficient transformation of the spiral arteries, impaired angiogenesis in the placenta and accumulation of type IV collagen, which subsequently leads to placental ischaemia and the development of PE [15].
The conducted study revealed an increased MMP-2 blood content in pregnant women who subsequently developed PE at 11–13 weeks. The differences in the data can be explained by the fact that in this study the level of MMP-2 was assessed at 11–13 weeks and, probably, in this period of pregnancy, an incorrectly formed placenta already triggers endothelial dysfunction and MMP-2 is an early and sensitive marker thereof.
The data of Palei et al are consistent with the results of the present work and indicate that the levels of both MMP-2 and MPP-9 are abnormally elevated starting from the first trimester in pregnant women, with the subsequent development of PE [16].
Martinez-Fierro et al conducted a study of urine MMP-2. They proved that its level rises starting from 12–16 weeks in patients with further development of PE [17]. An increase in MMP-2 concentration at 12 weeks predicts the development of PE with a sensitivity of 100% and specificity of 62.5%, and after 16 weeks with a sensitivity of 87.5% and a specificity of 74.1%.
Several works are devoted to the study of the practical application of the MMP level for predicting PE in the second and third trimesters of pregnancy. For example, Feng et al analysed not only the level of MMP-2 and MMP-9 but also their ratio in pregnant women in the second trimester from 20 weeks as potential biomarkers of PE [14]. They showed that MMP-2 and MMP-9 not individually but in the ratio of MMP-2/MMP-9 are significantly higher in pregnant women who will develop PE in the future.
The results of our study are consistent with the data of these authors. An increase in the level of MMP-2 and a decrease in MMP-9 was also found, which will undoubtedly lead to an increase in their ratio. However, a significant advantage of the present work is the assessment of these biomarkers starting from the first trimester at 11–13 weeks, simultaneously with ultrasound screening and uterine artery PI assessment.
It should be noted that few studies are examining the role of MMPs in the first trimester of pregnancy. Many of them were carried out in animal models; therefore it is necessary to continue the study of MMP-2 and MMP-9 as potential markers of the development of PE on wider clinical material.
Conclusion
In conclusion, it should be noted that the presented clinical study aimed to establish the value of MMP-2 and MMP-9 as biomarkers in early PE predicting, starting from the first trimester of pregnancy.
We found that the level of MMP-2 in the first trimester is significantly higher and the level of MMP-9 is significantly lower in pregnant women with the subsequent development of PE compared to uncomplicated pregnancy.
In the presented work, specific cut-off values of MMP-2 and MMP-9 are determined for predicting PE with high sensitivity and specificity.
The task of this work was to perform blood sampling from patients at 11–13 weeks of gestation and determine MMP plasma concentration. This term of entry is due to the fact that at this gestational age all pregnant women undergo prenatal screening, including the mandatory collection of blood samples.
Consequently, a weak point of this work may be highlighted, this being biomaterial sampling time. From 5 weeks to 11 weeks of gestation, the concentrations of MMP-2 and MMP-9 can change in parallel with the stages of placenta formation.
Therefore, the currently conducted study is devoted to the analysis of MMPs at 5–6, 11–13, and 18–19 weeks of gestation to establish the role of each MMP in the processes of implantation, invasion and gestational transformation of the spiral arteries.
The second pointed remark to our study is that the values of MMP are established as biomarkers of PE in general, without dividing it into early-onset PE and late-onset PE. In clinical practice, these are two different variants of the course of PE. Early PE is characterised by a higher incidence of life-threatening complications for the mother and foetus and turns out to be more resource-intensive. Therefore, differentiated prediction of early and late PE is relevant. Further study of the practical application of MMP in this matter has been planned. The specific prediction of both early and late PE and including other MMPs as well as their inhibitors into the study profile is promising.
Submitted/Doručeno: 14. 7. 2021
Accepted/Přijato: 16. 7. 2021
Prof. Elena Timokhina, MD
Department of Obstetrics, Gynecology and Perinatology
I. M. Sechenov First Moscow State Medical University
(Sechenov University)
8 Trubetskaya St.
119991 Moscow
Russian Federation
Publication ethics: The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Publikační etika: Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Conflict of interests: The authors declare they have no potential conflicts of interest concerning the drugs, products or services used in the study.
Konflikt zájmů: Autoři deklarují, že v souvislosti s předmětem studie/ práce nemají žádný konflikt zájmů.
Dedication: The study was funded by RFBR project number 19-315-90088.
Dedikace: Tato studie byla financována z projektu RFBR číslo 19-315-90088.
Sources
1. Poon LC, Shennan A, Hyett JA et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre‐eclampsia: a pragmatic guide for first‐trimester screening and prevention. Int J Gynaecol Obstet 2019; 145 (Suppl 1): 1–33. doi: 10.1002/ijgo. 12802.
2. Gestational hypertension and preeclampsia: ACOG practice bulletin, number 222. Obstet Gynecol 2020; 135 (6): e237–e260. doi: 10.1097/AOG.0000000000003891.
3. Timokhina EV, Strizhakov AN, Zafiridi NV et al. Innovative approach to prediction and therapy of preeclampsia: global experience. Akusherstvo I Ginekologiia 2019; 5: 5–10. doi: 10.18565/ aig.2019.5.5-10.
4. World Health Organization. Trends in maternal mortality 2000 to 2017: estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. Geneva: World Health Organization. 2019 [online]. Available from: https: //apps.who.int/iris/handle/10665/327596.
5. Li R, Tsigas EZ, Callaghan WM. Health and economic burden of preeclampsia: no time for complacency. Am J Obstet Gynecol 2017; 217 (3): 235–236. doi: 10.1016/j.ajog.2017.06.011.
6. Theilen LH, Meeks H, Fraser A et al. Long-term mortality risk and life expectancy following recurrent hypertensive disease of pregnancy. Am J Obstet Gynecol 2018; 219 (1): 107.e1–107.e6. doi: 10.1016/j.ajog.2018.04.002.
7. Lahti-Pulkkinen M, Girchenko P, Tuovinen S et al. Maternal hypertensive pregnancy disorders and mental disorders in children. Hypertension 2020; 75 (6): 1429–1438. doi: 10.1161/hyper- tensionaha.119.14140.
8. Bartsch E, Medcalf KE, Park AL et al. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ 2016; 353: i1753. doi: 10.1136/bmj.i1753.
9. Laskowska M. Altered maternal serum matrix metalloproteinases MMP-2, MMP-3, MMP-9, and MMP-13 in severe early- and late-onset preeclampsia. Biomed Res Int 2017; 2017: 6432426. doi: 10.1155/2017/6432426.
10. Su MT, Tsai PY, Tsai HL et al. miR-346 and miR-582-3p-regulated EG-VEGF expression and trophoblast invasion via matrix metalloproteinases 2 and 9. Biofactors 2017; 43 (2): 210–219. doi: 10.1002/biof.1325.
11. Sosa SE, Flores-Pliego A, Espejel-Nuñez A et al. New insights into the role of matrix metalloproteinases in preeclampsia. Int J Mol Sci 2017; 18 (7): 1448. doi: 10.3390/ijms18071448.
12. Erez O, Romero R, Maymon E et al. The prediction of late-onset preeclampsia: results from a longitudinal proteomics study. PLoS One 2017; 12 (7): e0181468. doi: 10.1371/journal.pone.0181468.
13. Plaks V, Rinkenberger J, Dai J et al. Matrix metalloproteinase-9 deficiency phenocopies features of preeclampsia and intrauterine growth restriction. Proc Natl Acad Sci USA 2013; 110 (27): 11109–11114. doi: 10.1073/pnas.1309561 110.
14. Feng H, Wang L, Zhang M et al. Ratio of matrix metalloproteinase-2 to -9 is a more accurate predictive biomarker in women with suspected pre-eclampsia. Biosci Re 2017; 37 (2): BSR20160508. doi: 10.1042/bsr20160508.
15. Lin C, He H, Cui N et al. Decreased uterine vascularization and uterine arterial expansive remodeling with reduced matrix -2 and -9 in hypertensive pregnancy. Am J Physiol Heart Circ Physiol 2020; 318 (1): H165–H180. doi: 10.1152/ajpheart.00602.2019.
16. Palei AC, Sandrim VC, Duarte G et al. Matrix metalloproteinase (MMP) -9 genotypes and haplotypes in preeclampsia and gestational hypertension. Clin Chim Acta 2010; 411 (11–12): 874–877. doi: 10.1016/j.cca.2010.03.002.
17. Martinez-Fierro ML, Perez-Favila A, Garza-Veloz I et al. Matrix metalloproteinase multiplex screening identifies increased MMP-2 urine concentrations in women predicted to develop preeclampsia. Biomarkers 2018; 23 (1): 18–24. doi: 10.1080/1354750x.2017.1279214.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicineArticle was published in
Czech Gynaecology

2021 Issue 4
Most read in this issue
- Pelvic neuropathic pain (differential diagnosis)
- HELLP syndrome and HELLP-like syndrome in pregnancies with covid-19 – case reports
- Molecular testing in endometrial carcinoma – joint recommendation of Czech Oncological Society, Oncogynecological Section of the Czech Gynecological and Obstetrical Society, Society of Radiation Oncology, Biology and Physics, and the Society of Czech Pathologists
- Recurrence of rare malignant Brenner ovarian tumor
