The role of the systemic inflammatory index in determining the length of hospital stay in patients with hyperemesis gravidarum
Úloha systémového zánětlivého indexu při určování délky hospitalizace u pacientů s hyperemesis gravidarum
Cíl: V této studii jsme se zaměřili na zjištění role parametrů periferní krve a indexu systémového zánětu (SII – systemic inflammatory index) v diagnostice hyperemesis gravidarum (HG) a zda mají prediktivní hodnotu při určování délky hospitalizace a rizika rehospitalizaci v případech HG. Materiál a metoda: V retrospektivní studii byly těhotné ženy hospitalizované pro HG (n = 112) a těhotné ženy zcela zdravé (n = 112) přiřazeny ke gestačnímu věku. Byly hodnoceny parametry zánětu periferní krve celé studované skupiny. Byla zaznamenávána délka hospitalizace a počet rehospitalizací pro případy HG. Výsledky: Do studie bylo zařazeno celkem 224 pacientů, 112 (50 %) v kontrolní skupině a 112 (50 %) ve skupině HG. Byla prokázána pozitivní korelace mezi zvýšenou ketonurií a délkou hospitalizace, parametry periferní krve a SII. Stupeň ketonurie byl shledán jako statisticky nevýznamný pro stanovení rizika rehospitalizace (p = 0,927). Do nemocnice byl přijato zpět 28,57 % (n = 32) případů HG. Při zohlednění délky hospitalizace bylo zjištěno, že SII je statisticky významný u hospitalizací trvajících déle než 2 dny (p = 0,001), ale nikoli u rehospitalizací (p = 0,3). Závěr: SII je významný v diagnostice a určování hospitalizace HG. Je dostačující pro stanovení délky hospitalizace, nikoli však rizika rehospitalizace, která je ukazatelem závažnosti onemocnění.
Klíčová slova:
hospitalizace – hyperemesis gravidarum – zánětlivé markery – systémový zánětlivý index
Authors:
Sukran Doğru 1
; Fatih Akkuş 1
; A. A. Atci 1
; M. Gümüş 2
; A. Acar 2
Authors‘ workplace:
Department of Obstetrics and Gynecology, Division of Perinatology, Necmettin Erbakan University Medical School of Meram, Konya, Turkey
1; Department of Obstetrics and Gynecology, Necmettin Erbakan University Medical School of Meram, Konya, Turkey
2
Published in:
Ceska Gynekol 2023; 88(3): 172-178
Category:
Original Article
doi:
https://doi.org/10.48095/cccg2023172
Overview
Objective: In this study, we aimed to investigate the role of peripheral blood parameters and the systemic inflammatory index (SII) in the diagnosis of hyperemesis gravidarum (HG) and whether they have a predictive value in determining the length of hospital stay and the risk of rehospitalization in HG cases. Materials and methods: In the retrospective study, pregnant women who were hospitalized due to HG (n = 112) and pregnant women who were completely healthy (n = 112) were matched for gestational age. Peripheral blood inflammation parameters of the entire study group were evaluated. The length of hospital stay and rehospitalization rate for HG cases were recorded. A total of 224 patients, 112 (50%) in the control group and 112 (50%) in the HG group were included in the study. There was a positive correlation between increased ketonuria and length of hospitalization, peripheric blood parameters, and SII. The degree of ketonuria was found to be statistically insignificant in determining the risk of rehospitalization (p = 0.927). About 28.57% (n = 32) of all HG cases were readmitted to the hospital. When the length of hospital stay was considered, SII was found to be statistically significant in hospitalizations lasting more than 2 days (p = 0.001), but not in rehospitalizations (p = 0.3). Conclusion: SII is significant in diagnosing and determining hospitalization of HG. It is sufficient to determine the length of hospital stay but not rehospitalization risk, which is an indicator of disease severity.
Keywords:
hospitalization – hyperemesis gravidarum – inflammation markers – systemic inflammatory index
Introduction
Nausea and vomiting (NVP) are among the most commonly seen clinical complaints in the first trimester of pregnancy. Approximately 70% of pregnant women experience NVP during this period [1]. On the other hand, hyperemesis gravidarum (HG) occurs when vomiting is severe and prolonged, which is a less common condition than NVP. Hyperemesis affects 1.5% of all pregnancies. It is the most frequent reason for admission to the hospital in early pregnancy and affects the well-being of pregnant women [2,3]. HG is characterized by persistent nausea, severe vomiting, electrolyte imbalance, acid-base imbalance, ketonuria (breakdown of fats due to decreased intake of carbohydrates as an energy source after a long fasting period), and loss of 5% or more of the initial gestational weight [4].
Although little is known about the etiology of HG, it is thought to be multifactorial and heterogeneous. Many factors are blamed for the etiopathogenesis of HG, such as human chorionic gonadotropin (b-HCG), estrogens, progesterone, thyroxine, elevated prolactin levels, decreased growth hormone, increased hypothalamic-hypophysis-adrenal axis activity, immunological mechanisms, gastrointestinal tract infection with Helicobacter pylori, decreased gastrointestinal motility, lower esophageal sphincter pressure and inflammation [5,6]. Differential diagnosis includes various infectious, endocrine, and gastrointestinal conditions. Therefore, there is a need for a sensitive test to support the clinical diagnosis, which has not been developed yet.
Many studies have been conducted on inflammatory markers in HG. Neutrophil/lymphocyte ratio (NLR), vaspin, C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-a), interleukin (IL) -6, and sirtuin-1 levels are found among the parameters associated with HG. It is known that TNF-a and IL-6, which are pro-inflammatory cytokines, are elevated in HG cases and induce subclinical inflammation. Various studies report significantly elevated NLR in HG cases, indicating disease severity [7]. In addition to their coagulation and hemostatic function, thrombocytes play an important role in regulating the inflammatory response. In cases of inflammation, megakaryocytes and their precursors increase in the bone marrow inducing a relative thrombocytosis, and lymphocyte counts decrease with increased apoptosis. All of which cause changes in peripheral blood inflammation parameters. There are studies in the literature on whether blood parameters predict the diagnosis of HG. There is no study on the role of these parameters in determining the length of a hospital stay.
In the present study, we aimed to investigate complete blood count parameters and the systemic inflammatory index (SII) in the diagnosis of HG and whether they have a predictive value for the length of hospitalization and rehospitalization risk.
Materials and methods
Retrospectively, between November 2021 and November 2022, 112 pregnant women were hospitalized at the Necmettin Erbakan University (NEU) Meram Medical Faculty Gynecology and Obstetrics Clinic due to HG. These women were matched one-to-one with 112 healthy pregnant women who were followed up on an outpatient basis. This study was approved by the NEU Ethics committee with a decision no: 2022/3617 (8261). The diagnosis of HG was made based on vomiting at least 3-times a day and a weight loss of 5% of the initial pregnancy weight. All patients with HG were selected from hospitalized patients. The control group consisted of pregnant women without any complaints matched for gestational age with the study group. The patients in the control group did not have nausea, vomiting, ketonuria, or weight loss. Control patients were not hospitalized at all. The entire control group consisted of ketone-negative cases. According to our clinic’s protocol, the severity of the disease is determined by ketonuria, the amount of vomiting, and the duration of hospitalization. When vomiting episodes stop, ketonuria is negative, and any deterioration in biochemical values improves, hospitalized patients are discharged. Patients who smoke, have multiple pregnancies, have suspected molar pregnancy or abortus imminens, thyroid disease, history of preeclampsia and diabetes, hepatic disease and psychiatric disorders, and patients receiving treatment for urinary tract infection and Helicobacter pylori were excluded from the study groups. Obstetric histories were obtained for all patients. Demographic characteristics were recorded. The gestational week for both groups was accepted as 5–18 weeks. Fetal heartbeats were positive. Complete blood count parameters at admission were recorded for both the HG and control groups. Length of hospital stay and ketonuria measurements were used to determine the severity of the disease in hyperemesis cases. In the HG subgroup analysis, the number of hospital admissions and length of hospital stays of HG cases were recorded. These groups were divided by considering the length of hospital stay of 2 days or less vs. more than 2 days. Urine samples of HG patients at admission were tested for ketone using a dipstick, which was then broken down as negative (0 mg/dl), 1+ (minor ketonuria; 0.5–15 mg/dl), 2+ (mild ketonuria; 15–40 mg/dl), 3+ (moderate ketonuria; 40–80 mg/dl), and 4+ (severe ketonuria; > 80 mg/dl). White blood cells (WBC), neutrophils (N), lymphocytes (L), monocytes (M), platelets (P), N/L (NLR), M/L (MLR), P/L (PLR), and SII (P × N/L) were measured. Blood samples were collected in ethylenediaminetetraacetate (EDTA) sterile tubes for all measurements. All measurements were made using the Mindray BC6200 automated blood count analyzer (Mindray Headquarters, China).
Statistical analysis
The data were analyzed using the Statistical Package for the Social Sciences (Version 22.0; IBM Corp., Armonk, NY, USA). Normality was determined by using the Kolmogorov-Smirnov test, the Shapiro-Wilk test, and histograms. The independent samples T-test was used to compare normally distributed variables between the HEG and control groups and the Mann-Whitney U-test was used if the variables were not normally distributed. The Kruskal-Wallis test was used to compare the MLR, NLR, PLR, SII, and LHS duration of HEG patients with different levels of ketonuria. Bonferroni corrected Mann-Whitney’s U-test was used to compare the ketonuria levels between the two groups. Comparison of inflammatory parameters and hospital stay according to rehospitalization was evaluated with the Mann-Whitney U-test. The Spearman correlation analysis was used to evaluate the relationship of ketonuria level with hemogram parameters, inflammation markers, and length of hospital stay.
Results
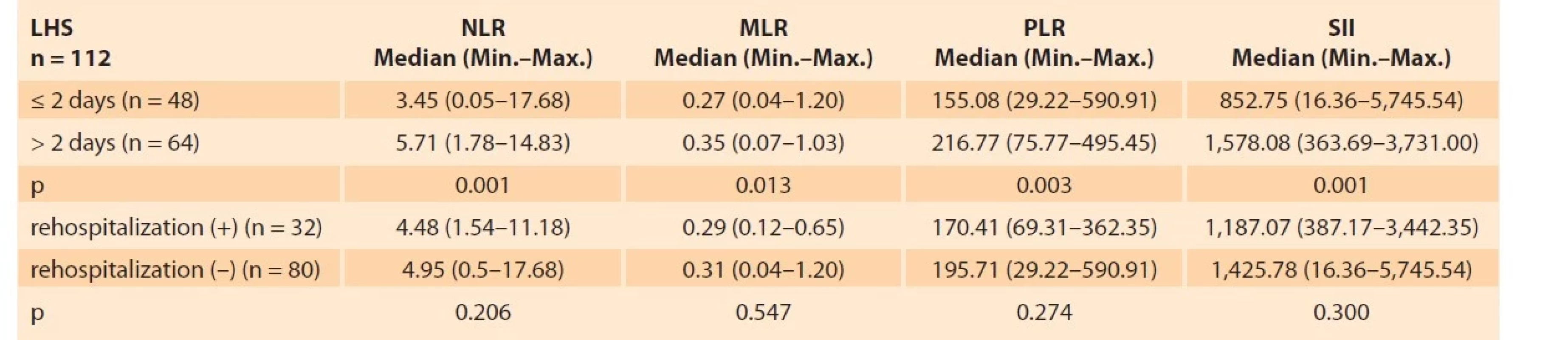
A total of 224 patients, 112 (50%) in the control group and 112 (50%) in the HG group were included in the study. Tab. 1 shows a comparison of the patients included in the study based on obstetric and hematological parameters. As shown in Tab. 1, the mean age, body mass index (BMI), M, NLR, MLR, PLR, and SII (p = 0.001; p = 0.015; p = 0.004; p = 0.0001; p = 0.0001; p = 0.0001; p = 0.0001; resp.) were significantly higher in the HG group compared to the control group, whereas L levels (p = 0.0001) were significantly lower. HG group and control group were similar to each other in terms of the gestational week, gravida, WBC, N, TSH (thyroid stimulating hormone), and P levels. In the HG group, ketonuria was +1 (22.4%) in 25 patients, +2 in 33 patients (29.4%), +3 in 29 patients (25.8%), and +4 in 25 (22.4%) patients. When blood parameters were evaluated according to ketonuria degrees in Tab. 2, NLR, PLR, MLR, and SII increased statistically significantly. The degree of ketonuria was found to be statistically insignificant in determining the risk of rehospitalization (p = 0.927). Tab. 3 provides the correlation between ketonuria and hemogram parameters, inflammation markers, and length of hospitalization. There was a positive correlation between increased ketonuria and length of hospitalization, WBC, P, M, N, NLR, PLR, MLR, and SII. However, there was a negative correlation between the increase in the degree of ketonuria and lymphocyte counts. Of the 112 patients, 48 (42.86%) stayed in the hospital for 2 days or less, and 64 (57.14%) stayed in the hospital for more than 2 days. When the length of hospital stay was considered, NLR, MLR, PLR, and SII were found to be statistically significant in hospitalizations lasting more than 2 days. About 28.57% (n = 32) of all HG cases were readmitted to the hospital. SII was not found to be statistically significant in determining these rehospitalized patients (p = 0.3) (Tab. 4).
Tab. 1. Porodnické a laboratorní parametry studijní a kontrolní skupiny.
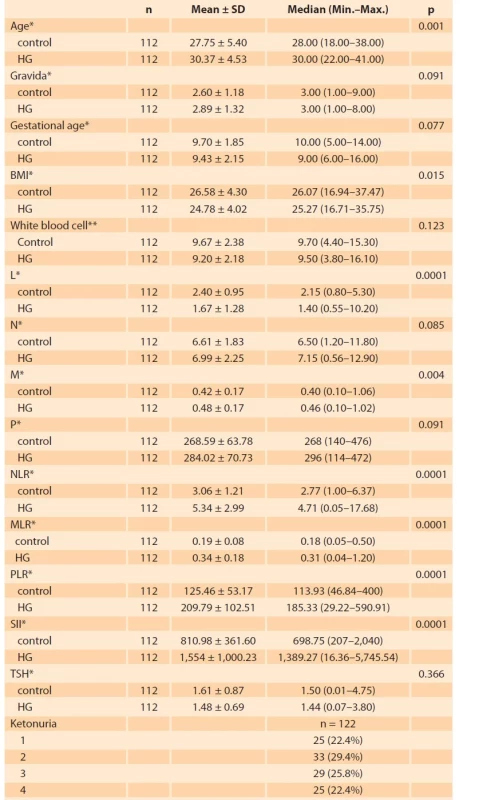
HG – hiperemesis gravidarum, L – lymphocyte, N – neutrophıl, M – monocyte, NLR – neutrophil/lymphocyte ratio, MLR – monocyte/lymphocyte ratio, P – platelet, PLR – platelet/lymphocyte
ratio, SII – systemic infl ammatory index, TSH – thyroid stimulant hormone
Tab. 2. Parametry hemogramu vycházející ze stupně ketonurie.
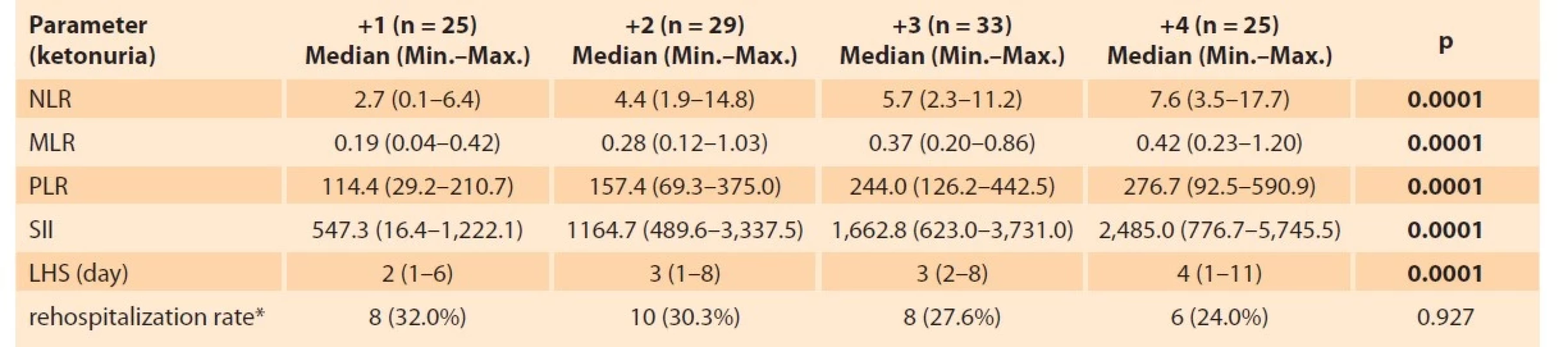
LHS – length of hospital stay (days), NLR – neutrophil/lymphocyte ratio, MLR – monocyte/lymphocyte ratio, PLR – platelet/lymphocyte ratio,
SII – systemic inflammatory index, TSH – thyroid stimulant hormone
Tab. 3. Vztah mezi ketonurií a charakteristikami pacienta, parametry hemogramu. Markery zánětu a délka hospitalizace.

L – lymphocyte, LHS – length of hospital stay (days), N – neutrophıl, NLR – neutrophil/lymphocyte ratio, M – monocyte, MLR – monocyte/lymphocyte ratio, P – platelet, PLR – platelet/lymphocyte ratio, SII – systemic inflammatory index, WBC – white blood cell
Tab. 4. Parametry hemogramu podle délky hospitalizace a rehospitalizačního stavu.
ROC analysis to evaluate the cut-off value of SII, NLR, PLR, and MLR to predict hospitalization for more than 2 days in pregnant women hospitalized for HEG is shown in Fig. 1. The SII cut-off value was 996,40, with sensitivity and specificity of 84% and 58%, resp. (AUC: 0.723; 95% CI 0.621–0.824; p = 0.0001). The cut-off value of NLR was 4.222, with sensitivity and specificity of 75% and 60%, resp. (AUC: 0.698; 95% CI 0.598–0.798; p = 0.0001). The cut-off value for PLR was 168,000. Sensitivity and specificity were 68% and 58%, respectively (AUC: 0.665; 95% CI 0.562–0.768; p = 0.003). With 64% sensitivity and 60% specificity (AUC: 0.638; 95% CI 0.532–0.743; p = 0.013), the MLR cut-off value was 0.30.
Obr. 1. Křivky provozních charakteristik přijímače pro systémový zánětlivý index (a), poměr neutrofilů/lymfocytů (b),
poměr krevních destiček/lymfocytů (c), poměr monocyt/lymfocytů (d) pro délku hospitalizace u případů hyperemesis
gravidarum.

Discussion
In this retrospective study, we aimed to examine whether peripheral blood parameters and SII predict the length of hospital stay and the risk of rehospitalization in patients with HG. These markers were significantly higher between HG patients and controls. We found that they predicted the length of the hospital stay but not rehospitalization. Although many available studies report a relationship between inflammatory markers and ketonuria in HG cases, no studies evaluate their effects on the length of hospital stay, which indicates disease severity. Previous studies showed that the rate of rehospitalization in pregnant women with HG was 19.2–30%. Cases that were hospitalized for more than two days exhibited a significantly higher risk of rehospitalization. These results show that cases with recurrent hospitalization had a longer stay during the first time [8]. Rehospitalization rates were evaluated in a study that included 10,380 outpatients and inpatients with HG. Readmission occurred in 60% of pregnancies, and inpatient readmission in 17% [9]. These findings raise the need to understand rehospitalization risk factors and develop a prognostic index.
There are conflicting data regarding the prediction of HG severity via ketonuria. A review of the literature reveals that only a small portion of patients with HG had ketonuria in available studies, of which a 2006 study reported a significant correlation between long-term hospital stay and ketonuria (these literature data are consistent with our study), while the other two studies reported no relationship between ketonuria and rehospitalization. The retrospective analysis of 192 HG cases revealed no biochemical markers, including white blood cell count, to predict rehospitalization [10,11]. We found a relationship between inflammatory markers and ketonuria in this study. In some studies, ketonuria was not associated with inflammatory markers [12], while Sosyal et al reported that NLR, PLR, and MLR increased with higher ketonuria levels in their study [13].
The role of inflammation in the etiology of HG has not been clarified yet. Available studies on inflammation markers support the existence of this relationship in these patients. Trophoblast-derived TNF-a regulates the production and release of human chorionic gonadotropin, interleukin (IL) -1, and IL-6. TNF-a stimulates placental prostaglandin E2, which peaks between weeks 9 and 12. Prostaglandin E2 and TNF-a levels are shown to be higher in maternal serum during periods of nausea and vomiting [14]. Akar et al discovered that NLR and PLR levels were significantly higher in HG cases compared to the control group [12]. Kan et al found that NLR, MLR, and PLR levels were significantly higher in HG cases [15]. Although thrombocytes are traditionally seen as key players in thrombosis and hemostasis and neutrophils as prime inflammatory cells, recent data demonstrate a complex interplay between these cells with important immune functions for thrombocytes and a clear role of neutrophils in thrombotic diseases. The mechanisms by which the thrombocyte-neutrophil interplay contributes to important infectious and thrombotic pathologies are only beginning to be explored, as is the role of these interactions in organ injury and repair [16]. NLR is a marker for the general immune response to various stress stimuli. Under inflammatory conditions, neutrophil precursors called myelocytes and promyelocytes may be released. A decrease in the number and function of lymphocytes may weaken the immunological defenses. Therefore, decreased lymphocyte count indicates cellular immune damage, while increased neutrophil and thrombocyte count is considered as a response to systematic inflammation [17]. In our study, SII, PLR, NLR, and MLR were significantly higher in HG cases, supporting these data.
SII is a new marker that has been developed to predict disease prognosis, particularly in cancer patients. Several studies have found it to be related to the severity of inflammatory diseases and infections [18]. SII has only been used in a few obstetric studies. Tanacan et al discovered a link between high SII values and poor newborn outcomes in pregnant women who suffered from premature rupture of membranes [19]. Örgül et al reported that systemic inflammatory indices (NLR, PLR, and SII) increased after starting treatment in pregnant women who were given a neuroprotective loading dose of magnesium sulfate [20]. In another study, NLR, PLR and MLR scores were found to be significantly higher in patients with preterm delivery compared to patients without preterm delivery, but they did not show a significant difference between SII study groups [21]. In the study of Dal et al, while NLR was significant in the group with HG compared to the control group, SII was not significant [22]. SII was predictive in our study for both the diagnosis of HG and the length of hospital stay.
A limited number of hospitalized HG patients, single-center and retrospective design, and the absence of a detailed analysis of cytokine responses were among the limitations of our study.
Conclusion
Inflammation markers and SII are effective in the diagnosis of HG. They are sufficient in predicting ketonuria and the length of hospitalization but not rehospitalization risk. The key role of inflammation in HG pathophysiology cannot be denied. We think that detailed studies on cytokines will make important contributions.
Authors’ Responsibility Statement
SD: Study design, patient management, and manuscript writing/editing; FA: Data analysis, patient management. AAA: Data analysis, patient management; MG: Data collection. AA: Contributed to and approved of the final version of the manuscript.
ORCID authors
Ş. Doğru 0000-0002-3383-2837
F. Akkuş 0000-0001-7037-9165
A. A. Atci 0000-0002-2637-3150
M. Gümüş 0000-0002-2964-3718
A. Acar 0000-0002-9074-258X
Submitted/Doručeno: 6. 2. 2023
Accepted/Přijato: 16. 3. 2023
Şükran Doğru, MD
Department of Obstetrics and Gynecology
Division of Perinatology
Necmettin Erbakan University Medical School
Yunus Emre, Akyokuş Street
42080 Meram/Konya
Turkey
Sources
1. Fejzo MS, Trovik J, Grooten IJ et al. Nausea and vomiting of pregnancy and hyperemesis gravidarum. Nat Rev Dis Primers 2019; 5 (1): 62. doi: 10.1038/s41572-019-0110-3.
2. Dean CR, Shemar M, Ostrowski GA et al. Management of severe pregnancy sickness and hyperemesis gravidarum. BMJ 2018; 363: k5000. doi: 10.1136/bmj.k5000.
3. Austin K, Wilson K, Saha S. Hyperemesisgravidarum. Nutr Clin Pract 2019; 34 (2): 226–241. doi: 10.1002/ncp.10205.
4. Boelig RC, Barton SJ, Saccone G et al. Interventions for treating hyperemesis gravidarum: a Cochrane systematic review and meta-analysis. J Matern Fetal Neonatal Med 2018; 31 (18): 2492–2505. doi: 10.1080/14767058.2017.1342805.
5. Agmon N, Sade S, Pariente G et al. Hyperemesis gravidarum and adverse pregnancy outcomes. Arch Gynecol Obstet 2019; 300 (2): 347–353. doi: 10.1007/s00404-019-05192-y.
6. Tayfur C, Burcu DC, Gulten O et al. Association between platelet to lymphocyte ratio, plateletcrit and the presence and severity of hyperemesis gravidarum. J Obstet Gynaecol Res 2017; 43 (3): 498–504. doi: 10.1111/jog.13228.
7. Aslan MM, Yeler MT, Bıyık İ et al. Hematological parameters to predict the severity of hyperemesis gravidarum and ketonuria. Rev Bras Ginecol Obstet 2022; 44 (5): 458–466. doi: 10.1055/s-0042-1743101.
8. Morris ZH, Azab AN, Harlev S et al. Developing and validating a prognostic index predicting re-hospitalization of patients with Hyperemesis Gravidarum. Eur J Obstet Gynecol Reprod Biol 2018; 225: 113–117. doi: 10.1016/j.ejogrb.2018.04.028.
9. Nurmi M, Rautava P, Gissler M et al. Readmissions due to hyperemesis gravidarum: a nation-wide Finnish register study. Arch Gynecol Obstet 2022; 306 (5): 1519–1529. doi: 10.1007/s00404-022-06448-w.
10. Tan PC, Jacob R, Quek KF et al. Readmission risk and metabolic, biochemical, haematological and clinical indicators of severity in hyperemesis gravidarum. Aust N Z J Obstet Gynaecolo 2006; 46 (5): 446–450. doi: 10.1111/j.1479-828X.2006.00632.x.
11. Niemeijer MN, Grooten IJ, Vos N et al. Diag- nostic markers for hyperemesis gravidarum: a systematic review and metaanalysis. Am J Obstet Gynecol 2014; 211 (2): 150.e151–150.e115. doi: 10.1016/j.ajog.2014.02.012.
12. Çintesun E, Akar S, Gul A et al. Subclinical inflammation markers in hyperemesis gravidarum and ketonuria: a case-control study. J Lab Physicians 2019; 11 (2): 149–153. doi: 10.4103/JLP.JLP_151_18.
13. Soysal C, Işıkalan MM, Bıyık İ et al. The relationship between inflammation markers and ketonuria in hyperemesis gravidarum. J Obstet Gynaecol Res 2021; 47 (9): 3078–3083. doi: 10.1111/jog.14857.
14. National Guideline Aliance. NICE Evidence Reviews Collection. In: Management of nausea and vomiting in pregnancy: antenatal care. UK: London: National Institute for Health and Care Excellence (NICE) 2021.
15. Kan E, Emektar E, Corbacioglu K et al. Evaluation of relationship between inflammatory markers and hyperemesis gravidarum in patients admitted to emergency department. Am J Emerg Med 2020; 38 (2): 292–295. doi: 10.1016/j.ajem.2019.05.007.
16. Lisman T. Platelet-neutrophil interactions as drivers of inflammatory and thrombotic disease. Cell Tissue Res 2018; 371 (3): 567–576. doi: 10.1007/s00441-017-2727-4.
17. Huang H, Liu Q, Zhu L et al. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci Rep 2019; 9 (1): 3284. doi: 10.1038/s415 98-019-39150-0.
18. Zhong JH, Huang DH, Chen ZY. Prognostic role of systemic immune-inflammation index in solid tumors: a systematic review and meta-analysis. Oncotarget 2017; 8 (43): 75381–75388. doi: 10.18632/oncotarget.18856.
19. Tanacan A, Uyanik E, Unal C et al. A cut-off value for systemic immune-inflammation index in the prediction of adverse neonatal outcomes in preterm premature rupture of the membranes. J Obstet Gynaecol Res 2020; 46 (8): 1333–1341. doi: 10.1111/jog.14320.
20. Orgul G, Agbal T, Celen S et la. Neuroprotective magnesium sulfate administration increases maternal Neutrophil-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio and Systemic Immune-Inflammation Index. Arch Gynecol Obstet 2021; 303 (6): 1433–1437. doi: 10.1007/s00404-020-05866-y.
21. Hrubaru I, Motoc A, Moise ML et al. The predictive role of maternal biological markers and inflammatory scores NLR, PLR, MLR, SII, and SIRI for the risk of preterm delivery. J Clin Med 2022; 11 (23): 6982. doi: 10.3390/jcm11236982.
22. Dal Y, Akkuş F, Karagün Ş et al. Are serum delta neutrophil index and other inflammatory marker levels different in hyperemesis gravidarum? J Obstet Gynaecol Res 2023; 49 (3): 828–834. doi: 10.1111/jog.15542.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicineArticle was published in
Czech Gynaecology

2023 Issue 3
Most read in this issue
- Pelvic pain in women after childbirth and physiotherapy
- Physiotherapy in a patient with diastasis of the rectus abdominis muscle after childbirth
- Prevention of intrauterine adhesions
- Obesity and assisted reproduction
